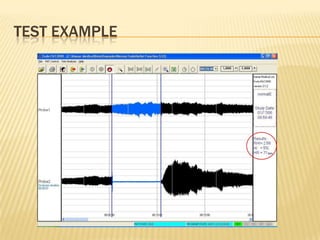
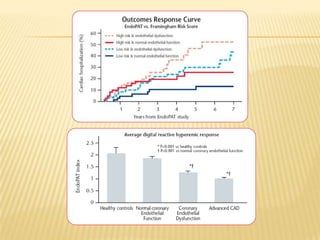
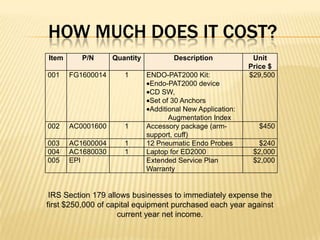
The document describes an FDA-approved, CE-marked device called the EndoPAT 2000 that non-invasively measures endothelial function to detect early signs of heart disease. The device uses fingertip probes to measure endothelial function through a 15-minute test that can be administered in a doctor's office. Benefits include increasing office revenue, improving patient care through early detection of risk factors, and monitoring treatment effectiveness. The device costs $250,000 but can provide a profit within 4 months for practices performing a modest number of tests per day.