Embed presentation
Download to read offline

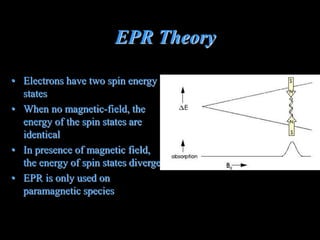
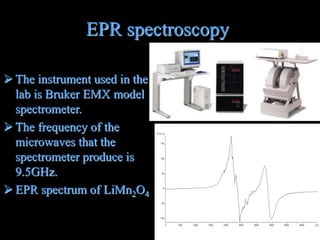

EPR spectroscopy uses microwaves and a magnetic field to measure the energy levels of unpaired electrons in paramagnetic materials. The document discusses EPR theory, describing how electron spin states diverge in a magnetic field. It also details the specific EPR spectrometer used in the author's lab, including its 9.5GHz microwave frequency. Finally, it explains how EPR can test battery materials like those used in the lithium-ion batteries developed for Mars rovers.



