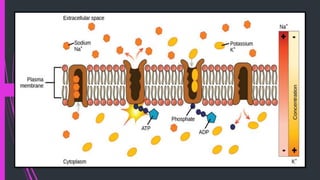
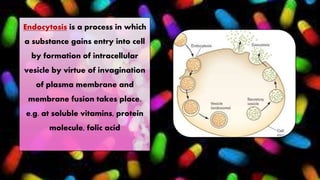
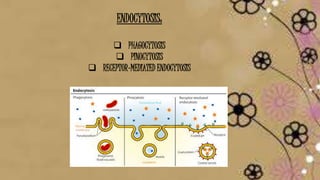
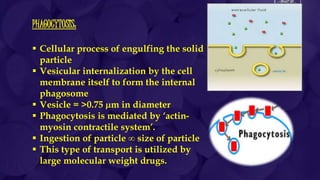
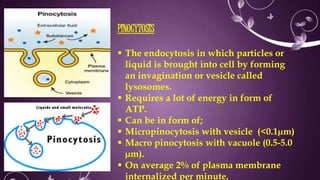
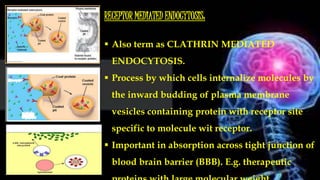
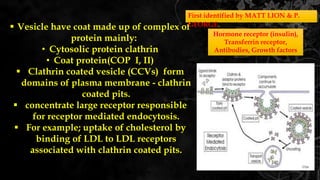
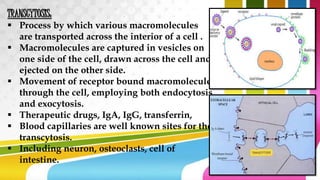
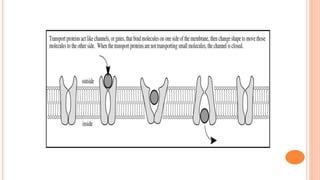
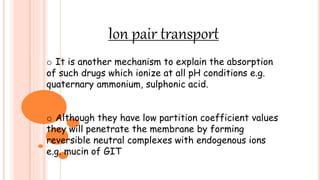
The document provides information about drug absorption. It defines drug absorption and discusses the processes that determine absorption such as passive transport, active transport, endocytosis, and transcytosis. It describes factors that affect drug absorption such as molecular size, degree of ionization, physical form, chemical nature, concentration, dosage forms, pH, and other substances in the gastrointestinal tract. The key points are that drug absorption involves movement of the drug into the bloodstream and is determined by the drug's properties and route of administration.