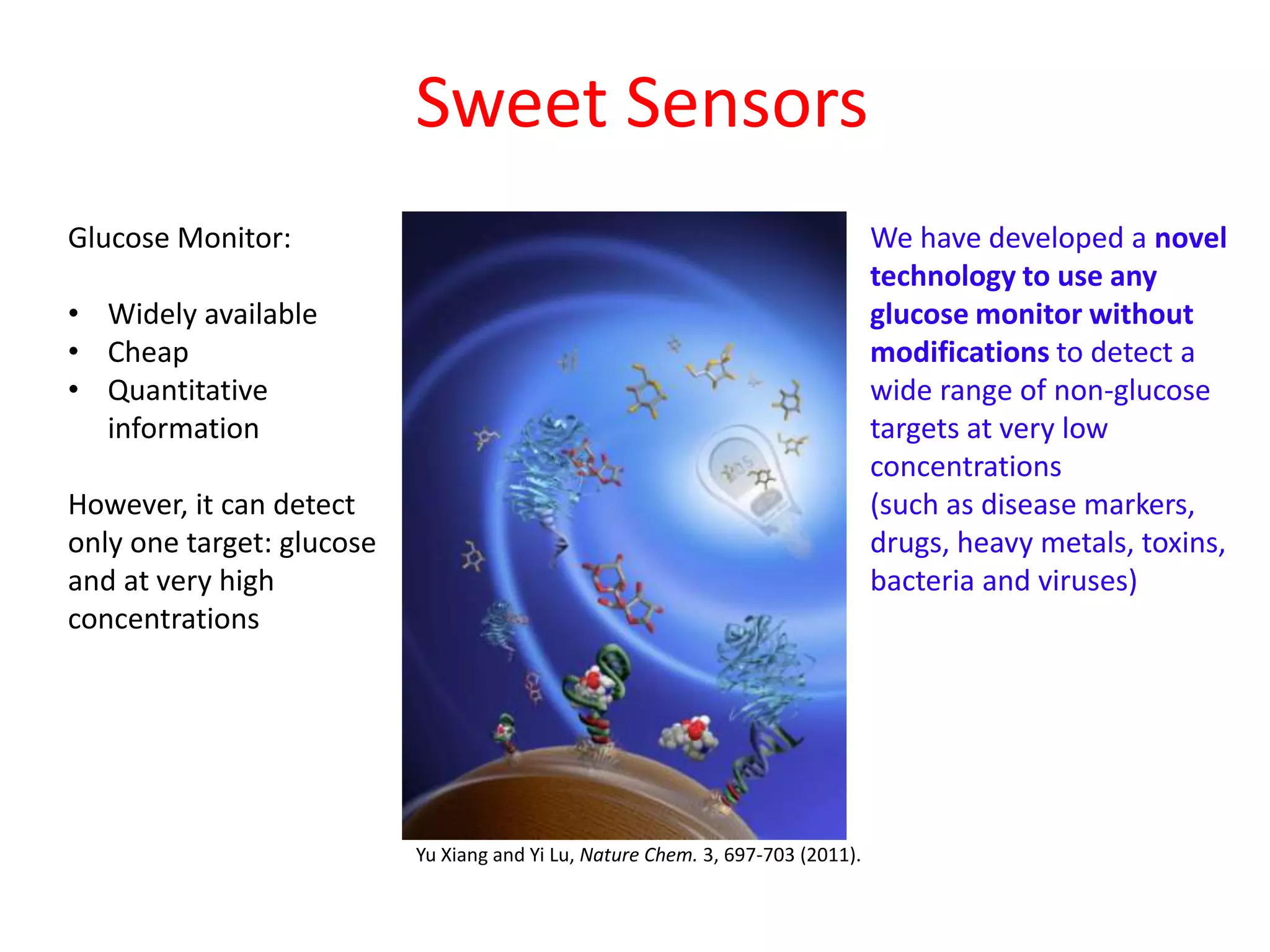
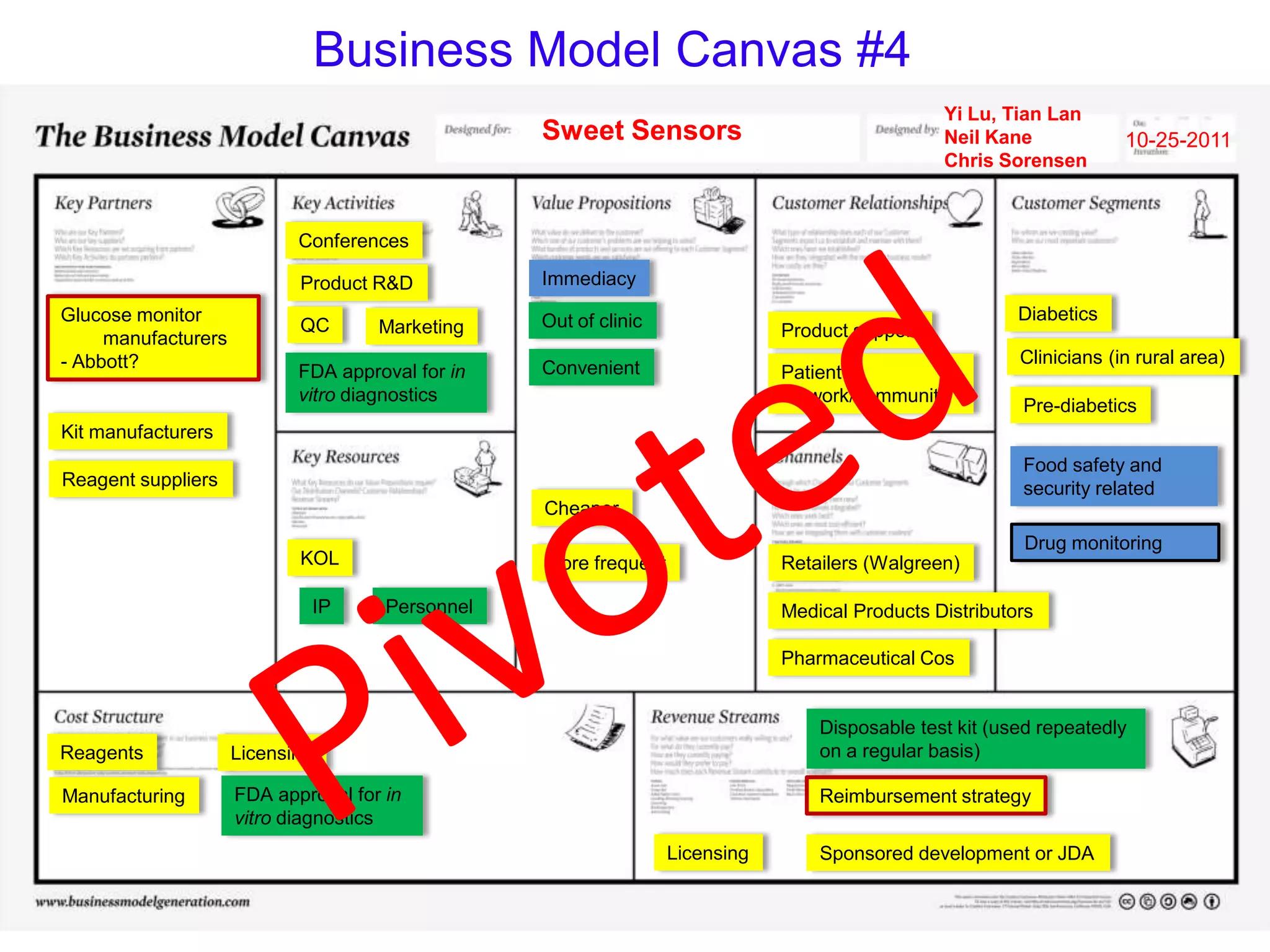
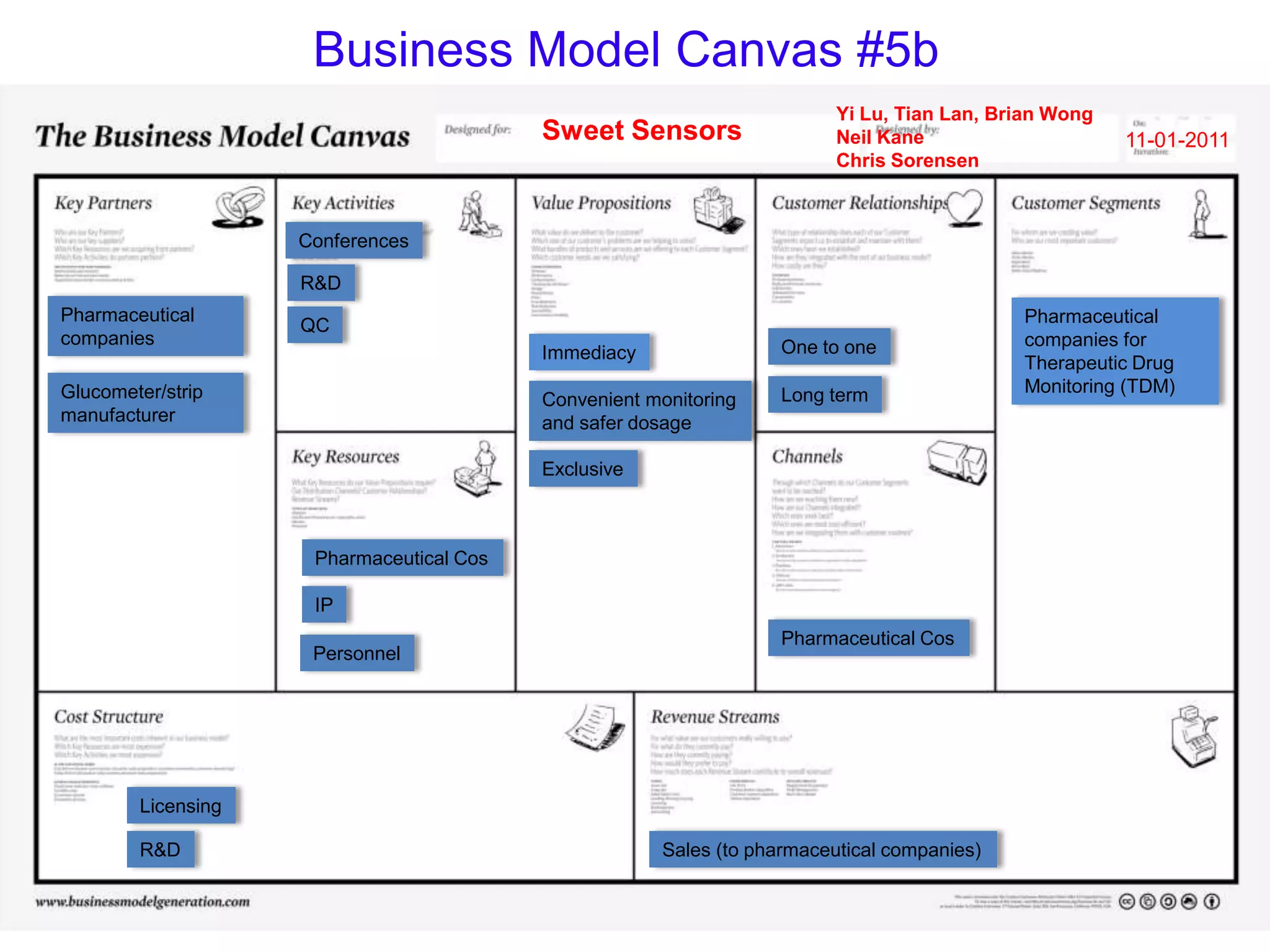
Sweet Sensors has developed a novel technology that allows any glucose monitor to detect a wide range of non-glucose targets like disease markers, drugs, toxins, and bacteria at very low concentrations without any modifications. However, existing glucose monitors can only detect glucose and only at high concentrations. The company is exploring business models focused on partnering with pharmaceutical companies for therapeutic drug monitoring and using alternative samples like saliva that do not require blood.