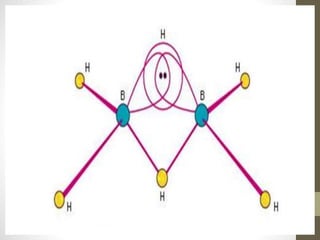
This document provides information on silicones and boron hydrides. It discusses the properties and applications of silicones, including their resistance to heat and chemicals, water repellency, electrical insulation, and strength. It also summarizes the classification of boron hydrides into three series and describes diborane as the most important boron hydride. The structure of diborane is explained, noting that it has an electron deficient structure and that its bonds involve both two-center two-electron bonds and three-center two-electron "banana" bonds between the boron and hydrogen atoms.