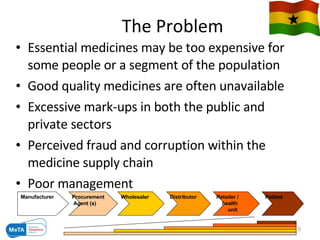
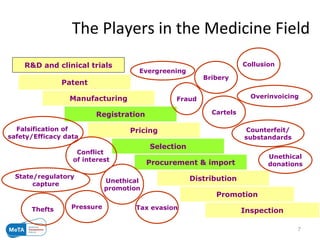
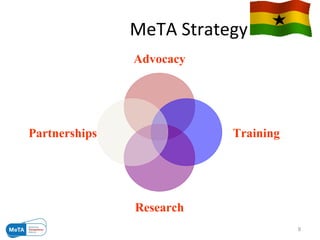
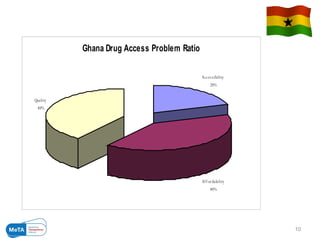
The Medicines Transparency Alliance (META) in Ghana aims to enhance transparency in the registration, procurement, distribution, and sales of essential medicines, addressing issues like high costs and quality availability. Key strategies include advocacy, training, research, and the establishment of a governing council to oversee the initiative's implementation. By improving access to affordable medicines and reducing corruption, META seeks to set a leadership example in West Africa for effective governance in health markets.