This document provides information on the global epidemiology and transmission of COVID-19. It discusses trends in cases and deaths globally and in the US. It reviews proposed routes of transmission, including via aerosols, droplets, fomites, and the environment. The viability of SARS-CoV-2 on different surfaces is summarized. Prevention strategies like hand washing, social distancing and face coverings are also covered.


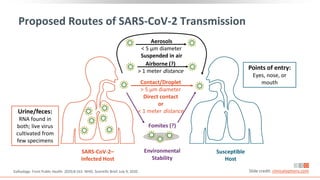
![Key Considerations on Modes of SARS-CoV-2
Transmission
Person-to-person considered predominant mode of transmission, likely via
respiratory droplets from coughing, sneezing, or talking[1,2]
‒ High-level viral shedding evident in upper respiratory tract[3,4]
‒ Airborne transmission suggested by multiple studies, but frequency unclear in
absence of aerosol-generating procedures in healthcare settings[2]
Virus rarely cultured in respiratory samples > 9 days after symptom onset,
especially in patients with mild disease[5]
Multiple studies describe a correlation between reduced infectivity with decreases
in viral loads and rises in neutralizing antibodies[5]
ACOG: “Data are reassuring that vertical transmission appears to be uncommon”[6]
1. https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/how-covid-spreads.html 2. WHO. Scientific Brief. July 9, 2020.
3. Wölfel. Nature. 2020;581:465. 4. Zou. NEJM. 2020;382:1177.
5. WHO. Scientific Brief. June 17, 2020. 6. ACOG. Practice Advisory: Novel Coronavirus 2019 (COVID-19). Last updated July 1, 2020. Slide credit: clinicaloptions.com
Updated
Updated](https://image.slidesharecdn.com/covid-19-230320060249-76fed1bc/85/Covid-19-pdf-9-320.jpg)
![Timing of SARS-CoV-2 Transmission
Based on Symptoms
Prospective study of lab-confirmed
COVID-19 cases (n = 100) and their close
contacts (n = 2761) in Taiwan[1]
‒ Paired index-secondary cases (n = 22)
occurred more frequently with exposure
just before or within 5 days of symptom
onset vs later
Pre-symptomatic infections
‒ Accounted for 6.4% of locally acquired
infections in a study in Singapore (N =
157)[2]
‒ Modelling study of transmission in China
(n = 154) estimated that 44% of
transmissions may have occurred just
before symptoms appeared[3]
A recent systematic review and meta-
analysis estimated that the proportion of
total infections that are truly
asymptomatic range from 6% to 41%
(pooled estimate of 15%)[4]
‒ Asymptomatic transmission rates ranged
from 0% to 2.2% vs symptomatic
transmission rates of 0.8% to 15.4%
‒ 3 studies reported that the cycle
threshold from RT-PCR assays did not
differ between symptomatic and
asymptomatic individuals
1. Cheng. JAMA Intern Med. 2020;[Epub]. 2. Wei. MMWR. 2020;69:411.
3. He. Nature Medicine. 2020;26:672. 4. Byambasuren. MedRxiv. 2020;[Preprint]. Note: this study has not been peer reviewed. Slide credit: clinicaloptions.com](https://image.slidesharecdn.com/covid-19-230320060249-76fed1bc/85/Covid-19-pdf-10-320.jpg)
![Nonpharmacologic Preventative Interventions
Inactivation of SARS-CoV, MERS-CoV,
and other endemic human
coronaviruses readily accomplished
with 62% to 71% ethanol, 0.5%
hydrogen peroxide, or 0.1% sodium
hypochlorite (in 1 min)[5]
‒ 0.05% to 0.2% benzalkonium
chloride, 0.02% chlorhexidine
digluconate less effective
1. https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/prevention.html 2. WHO. Scientific Brief. July 9, 2020.
3. Leung. Nat Med. 2020;26:676. 4. Chu. Lancet. 2020;395:1973. 5. Kampf. J Hosp Infect. 2020;104:246. Slide credit: clinicaloptions.com
Recommended Prevention Strategies[1,2]
Identify and quickly test suspect cases with
subsequent isolation of infected individuals
Quarantine close contacts of infected individuals
Wash hands often with soap and water
Maintain social distance (~ 6 feet)
Wear cloth face cover in public[3,4]
Practice respiratory etiquette
Disinfect frequent-touch surfaces regularly
Avoid crowds, close-contact settings, and poorly
ventilated spaces](https://image.slidesharecdn.com/covid-19-230320060249-76fed1bc/85/Covid-19-pdf-13-320.jpg)


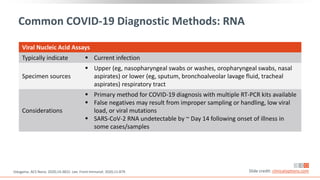

![CDC: Testing Recommendations for SARS-CoV-2
“Viral [nucleic acid or antigen] tests are recommended to diagnose acute
infection.”
Overview of Testing for SARS-CoV-2 (COVID-19). Updated August 24, 2020. https://www.cdc.gov/coronavirus/2019-
ncov/hcp/testing-overview.html Slide credit: clinicaloptions.com
Considerations for Who Should Get Tested
Persons with COVID-19 symptoms: test or self-isolate for ≥ 10 days after symptom onset and ≥ 24 hrs after
symptom resolution
Close contacts (within 6 ft) of known case for ≥ 15 mins: test if vulnerable individual or recommended by state
or local public health officials
No COVID-19 symptoms and no close contact with known case: no test needed
Persons in high transmission area who attended a gathering of ≥ 10 people without widespread mask wearing
or physical distancing: test if vulnerable individual or recommended by state or local public health officials
Work in a nursing home or long-term care facility: test regularly (frequency depends on incidence rate of area)
Live in or receive care in a nursing home or long-term care facility: test if symptomatic, if an outbreak occurs, or
routinely for persons who leave the facility on a regular basis
Critical infrastructure worker, health care worker, or first responder: may be tested according to employer’s
guidelines
Updated](https://image.slidesharecdn.com/covid-19-230320060249-76fed1bc/85/Covid-19-pdf-22-320.jpg)
![Temporal Profile of SARS-CoV-2 Viral Load
Serial viral loads assessed via
RT-PCR of posterior
oropharyngeal saliva or
endotracheal aspirate* collected
from hospitalized patients in
Hong Kong with laboratory
confirmed COVID-19 (N = 23)[1]
Viral loads highest during first
wk following symptom onset[1]
Pneumonia may develop late
and when URT PCR is negative[2]
*Intubated patients.
Slide credit: clinicaloptions.com
Mean
Viral
Load,
log
10
copies/mL
(SD)
Days After Symptom Onset
Temporal Viral Load in Patients With COVID-19[1]
10
8
6
4
2
0
0 10 20 30
Saliva
Endotracheal aspirate*
Day
Saliva
Endotracheal
0
1
0
1
1
0
2
3
0
3
3
0
4
5
0
5
5
0
6
5
0
7
4
0
8
7
1
9
10
0
10
5
1
11
8
2
12
7
2
13
7
2
14
6
2
15
7
2
16
8
2
17
5
2
18
6
2
19
5
2
20
6
2
21
6
1
22
5
1
23
6
1
24
4
1
25
3
1
26
3
0
27
2
0
28
2
0
29
1
0
1. To. Lancet Infect Dis. 2020;20:565. 2. Ai. Radiology. 2020;296:E32.](https://image.slidesharecdn.com/covid-19-230320060249-76fed1bc/85/Covid-19-pdf-24-320.jpg)
![P = .02
P < .001 P < .001
P < .001
Figure 3. A, Viral load of different tissue samples. B, Analysis of viral load in different clinical stages of ...
Viral load assessed via digital droplet PCR of samples collected from patients in
Beijing with laboratory confirmed COVID-19 (N = 76)
Viral Load Varies by Sample Type and Disease Stage
*Early stage: multifocal bilateral or isolated round ground-glass opacity with or without patchy consolidations and prominent peripherally
subpleural distribution on chest CT. Progressive stage: Increasing number, range, or density of lung lesions on chest CT. †Recovery phase:
lesions gradually absorbed. ‡Clinical cure: temperature recovery for > 3 days, improvement in respiratory symptoms, absorption of lung lesions,
and 2 consecutive negative RT-PCR results from respiratory samples tested at least 1 day apart.
Early and progressive stages*
(n = 15)
Recovery stage† (n = 49)
Clinical cure‡ (n = 5)
Slide credit: clinicaloptions.com
Mean
Viral
Load
(copies/test)
Mean
Viral
Load
(copies/test)
Type of Specimens
106
Nasal swabs
Throat swabs
Sputum
Blood
Urine
104
102
100
P < .001
Clinical Disease Stage
106
104
102
100
P < .001
Sputum Viral Load By Disease Stage
Viral Load By Sample Type
Yu. Clin Infect Dis. 2020;[Epub].](https://image.slidesharecdn.com/covid-19-230320060249-76fed1bc/85/Covid-19-pdf-25-320.jpg)

![Chest CT Abnormalities
Most common hallmark features on chest CT images include bilateral peripheral ground-
glass opacities and consolidations of the lungs with peak lung involvement between 6 days
and 11 days post-symptom onset[1-3]
In a study in Wuhan, China, chest CT imaging demonstrated a sensitivity of 97% and
specificity of 25% with RT-PCR as the reference (N = 1014)[4]
‒ 60% to 93% of patients had initial positive lung CT consistent with COVID-19 before the initial
positive RT-PCR result
1. Bernheim. Radiology. 2020;295:685. 2. Pan. Radiology. 2020;295:715. 3. Wang. Radiology. 2020;296:E55. 4. Ai. Radiology. 2020;296:E32. Slide credit: clinicaloptions.com
29-Yr-Old Man Presenting With Fever for 6 Days[4]
Ground-glass
opacities
Day 6 Day 9 Day 11 Day 17 Day 23](https://image.slidesharecdn.com/covid-19-230320060249-76fed1bc/85/Covid-19-pdf-27-320.jpg)
![SARS-CoV-2 in Stool
Reports of negative pharyngeal and sputum viral tests but fecal samples
testing positive for SARS-CoV-2[1]
Similar viral load but significantly longer duration of viral detection in stool
vs respiratory samples[2]
1. Chen. Am J Gastroenterol. 2020;115:790. 2. Zheng. BMJ. 2020;369:m1443. Slide credit: clinicaloptions.com
10
8
6
4
2
0
Respiratory Stool Serum
Viral
Load
(log
10
copies/mL)
P = .04 P < .001
P < .001
40
50
30
20
10
0
Respiratory Stool Serum
Days
After
Symptom
Onset
P = .02 P < .001
P = .07
60](https://image.slidesharecdn.com/covid-19-230320060249-76fed1bc/85/Covid-19-pdf-28-320.jpg)
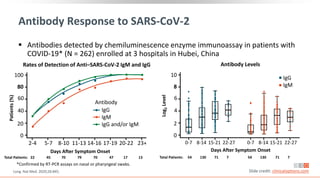
![Neutralizing Antibodies to SARS-CoV-2
Plasma collected from recovered
patients with COVID-19 who had
mild symptoms (N = 175)
Neutralizing antibody titers* varied
Neutralizing and spike-binding
antibodies emerged concurrently
between 10-15 days following
disease onset
Slide credit: clinicaloptions.com
Wu. medRxiv. 2020;[Preprint]. Note: This study has not been peer reviewed.
SARS-CoV-2
NAb
Titers
(ID50)
Disease Duration (Days)
*Assessed via pseudotyped, lentiviral vector-based assay.
NAb Titers Value (N = 175)
Titer range, ID50 < 40 to 21,567
Patients with undetectable level, % 6
Patients with detectable level, %
ID50: < 500 (very low)
ID50: 500-999 (medium low)
ID50: 1000-2500 (medium high)
ID50: > 2500 (high)
30
17
39
14
4096
2048
1024
512
256
128
64
32
16
2 4 6 8 10 12 14 161820 2224
Patient 1
Patient 2
Patient 3
Patient 4
Patient 5
Patient 6](https://image.slidesharecdn.com/covid-19-230320060249-76fed1bc/85/Covid-19-pdf-32-320.jpg)
![SARS-CoV-2 Serology for Diagnosis:
Current Recommendations
CDC: Given that it can take 1-3 wks to develop antibodies following infection, antibody test
results should not be used to diagnose someone with an active SARS-CoV-2 infection[1,2]
Royal College of Pathologists of Australasia[3]:
‒ “Molecular testing on a single throat with deep nasal swab is the current test of choice for the
diagnosis of acute COVID-19 infection”
‒ “COVID-19 IgG/IgM rapid tests have no role to play in the acute diagnosis of COVID-19 virus
infection . . . ”
‒ “COVID-19 IgG/IgM rapid tests will miss patients in early stages of disease when they are
infectious to other people”
WHO: “At present, based on current evidence, WHO recommends the use of these new
point-of-care immunodiagnostic tests only in research settings”[4]
Slide credit: clinicaloptions.com
1. https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antibody-tests-professional.html.
2. https://www.cdc.gov/coronavirus/2019-ncov/testing/serology-overview.html.
3. https://www.rcpa.edu.au/getattachment/bf9c7996-6467-44e6-81f2-e2e0cd71a4c7/COVID19-IgG-IgM-RAPID-POCT-TESTS.aspx.
4. https://www.who.int/news-room/commentaries/detail/advice-on-the-use-of-point-of-care-immunodiagnostic-tests-for-covid-19.](https://image.slidesharecdn.com/covid-19-230320060249-76fed1bc/85/Covid-19-pdf-33-320.jpg)

![COVID-19 Incubation: Infection to Illness Onset
Among 10 confirmed NCIP cases in
Wuhan, Hubei province, China[1]
‒ Mean incubation: 5.2 days
(95% CI: 4.1-7.0)
Among 181 confirmed SARS-CoV-2
infections occurring outside of Hubei
province[2]
‒ Median incubation: 5.1 days
(95% CI: 4.5-5.8)
‒ Symptom onset by Day 11.5 of
infection in 97.5% of persons
1. Li. NEJM. 2020;382:1199. 2. Lauer. Ann Intern Med. 2020;172:577. Slide credit: clinicaloptions.com
Estimated Incubation Period Distribution[1]
0.25
0.20
0.15
0.10
0.05
0
Relative
Frequency
Days From Infection to Symptom Onset
21
0 7 14](https://image.slidesharecdn.com/covid-19-230320060249-76fed1bc/85/Covid-19-pdf-35-320.jpg)
![COVID-19 Clinical Presentation May Vary by Age, Sex
Observational study of Europeans with mild-to-moderate
COVID-19 (ie, no ICU admission) via standardized
questionnaire during March 22-April 10, 2020 (N = 1420)[1]
‒ Mean duration of symptoms (n = 264): 11.5 ± 5.7 days
‒ Ear, nose, throat complaints more common in young patients;
fever, fatigue, loss of appetite, diarrhea in elderly patients
(P < .01)
‒ Loss of smell, headache, nasal obstruction, throat pain, fatigue
more common in women; cough, fever in men (P < .001)
Among 17 fatal COVID-19 cases detailed by the China
National Health Commission, median time from first
symptom to death: 14 days (range: 6-41)[2]
‒ Numerically faster in older patients: 11.5 days if ≥ 70 yrs vs 20
days if < 70 yrs (P = .033)
1. Lechien. J Intern Med. 2020;[Epub]. 2. Wang. J Med Virol. 2020;92:441. Slide credit: clinicaloptions.com
Symptom,[1] % N = 1420
Headache 70.3
Loss of smell 70.2
Nasal obstruction 67.8
Asthenia 63.3
Cough 63.2
Myalgia 62.5
Rhinorrhea 60.1
Taste dysfunction 54.2
Sore throat 52.9
Fever (> 38°C) 45.4](https://image.slidesharecdn.com/covid-19-230320060249-76fed1bc/85/Covid-19-pdf-37-320.jpg)


![Vaccine Development Pathway
Traditional vaccine development pathway[1]
‒ Target discovery/validation, preclinical stage, manufacturing development, clinical
assay optimization: 3-8 yrs
‒ Phase I (safety), phase II (safety/immunogenicity), phase III (safety/efficacy) clinical
trials: 2-10 yrs
‒ Regulatory review: 1-2 yrs
Slide credit: clinicaloptions.com
1. Heaton. NEJM. 2020;[Epub].
2. The New York Times. Coronavirus Vaccine Tracker. https://www.nytimes.com/interactive/2020/science/coronavirus-vaccine-tracker.html
SARS-CoV-2 Vaccine Candidates in Development[2]
Preclinical Phase I Phase II Phase III Approval
Vaccines not
yet in human trials
Vaccines testing
safety and dosage
Vaccines in expanded
safety trials
Vaccines in large-
scale efficacy
tests
Vaccine approved
for limited use
140+ 19 12 5 1](https://image.slidesharecdn.com/covid-19-230320060249-76fed1bc/85/Covid-19-pdf-44-320.jpg)
![Virus genes
(some inactive)
Vaccine Candidates in Development for SARS-Cov-2
Slide credit: clinicaloptions.com
Funk. Frontiers in Pharmacology. 2020;[Epub].
Vaccine Platforms Vaccine Candidates
44
16
14
10 12
20
3
8
Other DNA
RNA
SARS-CoV-2
inactivated
SARS-CoV-2
live
attenuated
Viral vector
(nonreplicating)
Viral vector
(replicating)
Protein based
Viral vector
(nonreplicating)
Viral vector
(replicating)
Virus
(inactivated)
Virus
(attenuated)
DNA
RNA (+ LNPs)
Protein based
(eg, spike)
Coronavirus
spike gene
Virus genes
(some inactive)
Coronavirus
spike gene](https://image.slidesharecdn.com/covid-19-230320060249-76fed1bc/85/Covid-19-pdf-45-320.jpg)
![mRNA Vaccine Against SARS-CoV-2:
Preliminary Safety Report
No serious adverse events reported
Slide credit: clinicaloptions.com
Jackson. NEJM. 2020;[Epub].
0
Symptom Dose Group Vaccination 1 Vaccination 2
Mild Moderate Severe
Any systemic
symptom
Arthralgia
Fatigue
Fever
Chills
Headache
25 μg
100 μg
250 μg
25 μg
100 μg
250 μg
25 μg
100 μg
250 μg
25 μg
100 μg
250 μg
25 μg
100 μg
250 μg
25 μg
100 μg
250 μg
Participants (%)
0
Symptom Dose Group Vaccination 1 Vaccination 2
Myalgia
Nausea
Any local
symptom
Size of erythema
or redness
Size of induration
or swelling
Pain
25 μg
100 μg
250 μg
25 μg
100 μg
250 μg
25 μg
100 μg
250 μg
25 μg
100 μg
250 μg
25 μg
100 μg
250 μg
25 μg
100 μg
250 μg
Participants (%)
100
40 60 80
20 100
40 60 80
20 100
40 60 80
20
100
40 60 80
20](https://image.slidesharecdn.com/covid-19-230320060249-76fed1bc/85/Covid-19-pdf-46-320.jpg)
![Dose and Age Considerations
Many phase III vaccine studies fail because the dose chosen did not
best balance safety and efficacy[1]
Immune function declines with age and this decline is likely partially
responsible for the greater risk of severe COVID-19 in older adults
may lead to poor vaccine responses and the need for higher vaccine
doses in older adults[2,3]
Slide credit: clinicaloptions.com
1. Musuamba. CPT Pharmacometrix Syst Pharmacol. 2017;6:418. 2. Heaton. NEJM. 2020;[Epub]. 3. Zhu. Lancet. 2020;[Epub].](https://image.slidesharecdn.com/covid-19-230320060249-76fed1bc/85/Covid-19-pdf-47-320.jpg)

![Medical Management of Mild COVID-19
1. WHO Interim Guidance. Clinical management of COVID-19. May 27, 2020.
2. NIH COVID-19 Treatment Guidelines. Management of persons with COVID-19. Last updated June 11, 2020.
3. NIH COVID-19 Treatment Guidelines. Antithrombotic therapy in patients with COVID-19. Last updated May 12, 2020. Slide credit: clinicaloptions.com
WHO[1]
Isolate suspected/confirmed cases to contain
SARS-CoV-2 transmission; isolation can occur
at home, in a designated COVID-19 health or
community facility
Treat symptoms (eg, antipyretics for fever,
adequate nutrition, appropriate rehydration)
Educate patients on signs/symptoms of
complications that, if developed, should
prompt pursuit of urgent care
NIH[2,3]
Majority of cases managed in ambulatory
setting or at home (eg, by telemedicine)
Close monitoring advised for symptomatic
patients with risk factors for severe disease;
rapid progression possible
No specific lab tests indicated if otherwise
healthy
In non-hospitalized patients, do not initiate
anticoagulants or antiplatelet therapy to
prevent VTE or arterial thrombosis unless
other indications exist](https://image.slidesharecdn.com/covid-19-230320060249-76fed1bc/85/Covid-19-pdf-49-320.jpg)
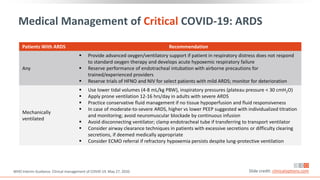
![Management[1]
Monitor closely, as pulmonary disease can
rapidly progress
Administer empiric antibiotics if bacterial
pneumonia/sepsis strongly suspected; re-
evaluate daily and de-escalate/stop treatment
if no evidence of infection
Use hospital infection prevention and control
measures; limit number of
individuals/providers entering patient room
Use AIIRs for aerosol-generating procedures;
staff should wear N95 respirators or PAPRs vs
surgical masks
Medical Management of Moderate COVID-19
Slide credit: clinicaloptions.com
Initial Evaluation[1]
May include chest x-ray, ultrasound, or CT
Perform ECG if indicated
Obtain CBC with differential and metabolic
profile including liver/renal function
Inflammatory markers (eg, CRP, D-dimer,
ferritin) may be prognostically valuable
Isolation (Home vs Healthcare Facility)[2]
Dependent on clinical presentation,
requirement for supportive care, presence of
vulnerable household contacts; if high risk of
deterioration, hospitalization preferred
1. NIH COVID-19 Treatment Guidelines. Management of persons with COVID-19. Last updated June 11, 2020.
2. WHO Interim Guidance. Clinical management of COVID-19. May 27, 2020.](https://image.slidesharecdn.com/covid-19-230320060249-76fed1bc/85/Covid-19-pdf-50-320.jpg)
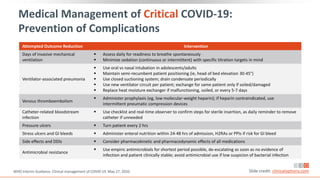
![Acute Coinfection Treatment[1]
Administer empiric antimicrobials within 1 hr of
initial assessment based on clinical judgment,
patient host factors, and local epidemiology;
knowledge of blood cultures before antimicrobial
administration ideal
Assess daily for antimicrobial de-escalation
Severe Pneumonia Treatment[1]
Equip patient care areas with pulse oximeters,
functioning oxygen systems, and disposable, single-
use, oxygen-delivering interfaces
Provide immediate supplemental oxygen to
patients with emergency signs (eg,
obstructed/absent breathing, severe respiratory
distress, central cyanosis, shock, coma, or
convulsions) and anyone with SpO2 < 90%
Monitor for clinical deterioration (eg, rapidly
progressive respiratory failure, shock); provide
immediate supportive care
Practice cautious fluid management in patients
without tissue hypoperfusion and fluid
responsiveness
Medical Management of Severe COVID-19
Slide credit: clinicaloptions.com
1. WHO Interim Guidance. Clinical management of COVID-19. May 27, 2020.
2. NIH COVID-19 Treatment Guidelines. Management of persons with COVID-19. Last updated June 11, 2020.
Evaluation[2]
Perform evaluations outlined for moderate disease](https://image.slidesharecdn.com/covid-19-230320060249-76fed1bc/85/Covid-19-pdf-51-320.jpg)
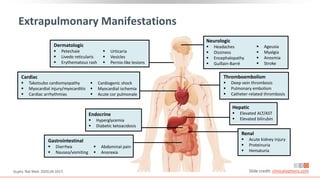
![≥ 24 hrs since resolution
of fever, last antipyretics
CDC: Discontinuation of Transmission-Based
Precautions in Symptomatic COVID-19 Patients
“A test-based strategy is no
longer recommended [except
for rare situations] because, in
the majority of cases, it results
in prolonged isolation of
patients who continue to shed
detectable SARS-CoV-2 RNA but
are no longer infectious.”
Symptom-Based Strategy
And
https://www.cdc.gov/coronavirus/2019-ncov/hcp/disposition-hospitalized-patients.html Slide credit: clinicaloptions.com
Improvement in symptoms
(eg, cough, shortness of breath)
And
≥ 10 days since symptom onset
for mild to moderate illness,
≥ 20 days for severe to critical illness
or those severely immunocompromised](https://image.slidesharecdn.com/covid-19-230320060249-76fed1bc/85/Covid-19-pdf-55-320.jpg)
![Burden of Thrombosis in Patients With COVID-19
Study Country Design Population N Thromboprophylaxis Screening VTE Rate, %
China[1] Retrospective ICU 81 No No 25.0
France[2] Prospective ICU 150 Yes No 11.7*
France[3] Retrospective ICU 26 Yes Yes 69.0
France[4] Retrospective ICU 107 Yes No 20.6†
The
Netherlands[5] Retrospective ICU 184 Yes No 27.0
Italy[6] Retrospective Inpatient 388 Yes No 21.0
United
Kingdom[7] Retrospective ICU 63 Yes No 27.0
*Pulmonary embolisms in COVID-19 ARDS vs 2.1% in matched non-COVID-19 ARDS.†Pulmonary embolism vs 6.1% in non–COVID-19 ICU patients.
1. Cui. J Throm Maemost. 2020;[Epub]. 2. Helms. Intesive Care Med. 2020;46:1089. 3. Llitjos. J Thromb Haemost. 2020;18:1743. 4. Poissy.
Circulation. 2020;142:184. 5. Klok. Throm Res. 2020;191:145. 6. Lodigiani. Thromb Res. 2020;191:9. 7. Thomas. Thromb Res. 2020;191:76. Slide credit: clinicaloptions.com
New](https://image.slidesharecdn.com/covid-19-230320060249-76fed1bc/85/Covid-19-pdf-56-320.jpg)
![Autopsy Evidence of Lung Damage in COVID-19
Prospective study to compare
clinical findings with data from
autopsy (N = 12)[1]
‒ 7/12 patients had unsuspected
bilateral DVT
‒ 4/7 died from PE
Alveolar Damage[2]
Organizing Microthrombus[2]
1. Wichmann. Ann Int Med. 2020;[Epub]. 2. Carsana. Lancet Infect Dis. 2020. doi.org/10.1016/S1473-3099(20)30434-5. Slide credit: clinicaloptions.com
New](https://image.slidesharecdn.com/covid-19-230320060249-76fed1bc/85/Covid-19-pdf-57-320.jpg)
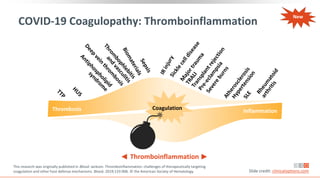
![Guidance on Thromboprophylaxis
NIH[1] ASH[2]
Hospitalized adults with COVID-19 should receive VTE
prophylaxis per the SoC for other hospitalized adults
Anticoagulant or antiplatelet therapy should not be
used to prevent arterial thrombosis outside of the usual
SoC for patients without COVID-19
Currently insufficient data to recommend for or against
the use of thrombolytics or increasing anticoagulant
doses for VTE prophylaxis in hospitalized COVID-19
patients outside of clinical trial
Hospitalized patients should not be routinely discharged
on VTE prophylaxis (extended VTE prophylaxis can be
considered in patients with low bleeding risk and high
VTE risk)
All hospitalized adults with COVID-19 should receive
thromboprophylaxis with low-molecular-weight
heparin over unfractionated heparin, unless bleeding
risk outweighs thrombosis risk
Fondaparinux is recommended in the setting of
heparin-induced thrombocytopenia
In patients in whom anticoagulants are contraindicated
or unavailable, use mechanical thromboprophylaxis
(eg, pneumatic compression devices)
Encourage participation on clinical trials rather than
empiric use of therapeutic-dose heparin in COVID-19
patients with no other indication for therapeutic dose
anticoagulation
Slide credit: clinicaloptions.com
1. NIH. COVID-19 Treatment Guidelines. Updated: May 12, 2020. 2. American Society of Hematology. COVID-19 and VTE/
Anticoagulation: Frequently Asked Questions. 3. Spyropoulos. J Thromb Haemost. 2020;[Epub]. 4. Moores. CHEST. 2020;[Epub].
*Additional recommendations available from the International Society on Thrombosis and Haemostasis[3], and CHEST.[4]
Recommending Organization*
New](https://image.slidesharecdn.com/covid-19-230320060249-76fed1bc/85/Covid-19-pdf-59-320.jpg)
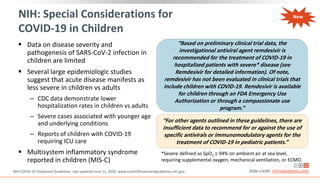
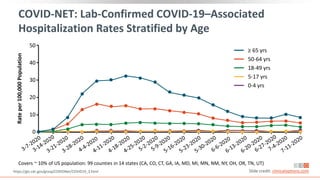
![COVID-19–Associated Hospitalization and
Death Rates Increase With Age in US
1. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/covid-net/purpose-methods.html.
2. https://www.cdc.gov/covid-data-tracker/index.html#demographics. Slide credit: clinicaloptions.com
*Lab-confirmed COVID-19 cases; covers ~ 10% of US population: 99 counties in 14 states (CA, CO, CT, GA, IA, MD, MI, MN, NM, NY, OH, OR, TN, UT).
†Data from 109,622 deaths in confirmed and probable COVID-19 cases as reported by US states and territories; age group data available for 109,602
deaths (99%).
COVID-19–Associated Hospitalizations*[1] COVID-19–Associated Deaths†[2]
%
of
Total
Deaths
Age Group (Yrs)
Rates
per
100,000
Population
Wk
400
350
300
250
200
150
100
50
0
0-4 yrs
5-17 yrs
18-49 yrs
50-64 yrs
65+ yrs
100
0 10 20 30 40 50 60 70 80 90
0-4: < 0.1%
5-17: < 0.1%
18-29: 0.5%
30-39: 1.3%
40-49: 3.1%
50-64: 15.4%
65-74: 20.9%
75-84: 26.4%
85+: 32.4%
New](https://image.slidesharecdn.com/covid-19-230320060249-76fed1bc/85/Covid-19-pdf-61-320.jpg)

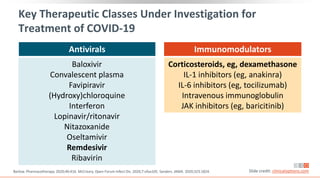

![Key RCT Data For Other Investigational Agents
Agent N Population Comparator Primary Outcome
Lopinavir/ritonavir[1] 199 Adults, severe SOC alone No difference in time to clinical improvement
Lopinavir/ritonavir[2] 86
Adults, mild-to-
moderate
Umifenovir or
no antiviral
No difference in rate of positive-to-negative
conversion of SARS-CoV-2 nucleic acid
Lopinavir/ritonavir +
ribavirin + IFNβ1b[3] 86 Adults, hospitalized LPV/RTV
Significantly shorter median time from start
of study treatment to negative
nasopharyngeal swab for combination
treatment
Lopinavir/ritonavir*[4] 1596 Hospitalized SOC alone No difference in 28-day mortality
Favipiravir*[5] 240 Adults, pneumonia Umifenovir No difference in clinical recovery rate of Day 7
Hydroxychloroquine*[6] 150
Adults, mild-to-
moderate
SOC alone
No difference in negative conversion of SARS-
CoV-2 by Day 28
Hydroxychloroquine*[7] 1542 Hospitalized SOC alone No difference in 28-day mortality
Slide credit: clinicaloptions.com
1. Cao. NEJM. 2020;382:1787. 2. Li. Med. 2020;[Epub]. 3. Hung. Lancet. 2020;395:1695.
4. https://www.recoverytrial.net/files/lopinavir-ritonavir-recovery-statement-29062020_final.pdf
5. Chen. https://doi.org/10.1101/2020.03.17.20037432 6. Tang. https://doi.org/10.1101/2020.04.10.20060558
7. https://www.recoverytrial.net/files/hcq-recovery-statement-050620-final-002.pdf
*Published as a preprint or by press release only; not yet peer-reviewed.](https://image.slidesharecdn.com/covid-19-230320060249-76fed1bc/85/Covid-19-pdf-67-320.jpg)
![Key RCT Data For Other Investigational Agents (Cont.)
Agent N Population Comparator Primary Outcome
Tocilizumab[1,2] 129
Moderate or
severe
pneumonia
Standard care
alone
Improvement in composite
endpoint of death or need for
ventilation at Day 14 with
tocilizumab vs standard care
Sarilumab
(200 or 400 mg)[3,4] 457 Severe or critical Placebo
CRP decline: 77% and 79% vs 21%
IDMC recommended continuing
phase III only in critical subgroup
with 400 mg sarilumab vs placebo
Slide credit: clinicaloptions.com
1. https://www.aphp.fr/contenu/tocilizumab-improves-significantly-clinical-outcomes-patients-moderate-or-severe-covid-19
2. NCT04331808. 3. NCT04315298. 4. https://newsroom.regeneron.com/news-releases/news-release-details/regeneron-and-
sanofi-provide-update-us-phase-23-adaptive](https://image.slidesharecdn.com/covid-19-230320060249-76fed1bc/85/Covid-19-pdf-68-320.jpg)
