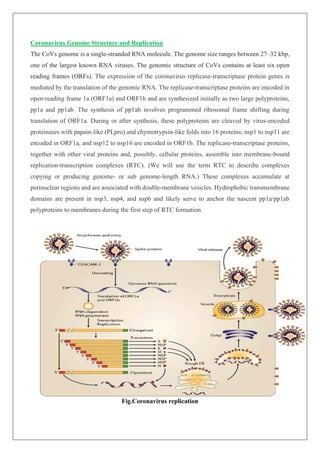
The document discusses the coronavirus (COVID-19), detailing its structure, classification, and the impact on global health and economies since its emergence in late 2019. It outlines symptoms, treatments like plasma therapy and medications such as remdesivir and dexamethasone, and explores vaccine development methods, including inactivated, DNA, and RNA-based approaches. The conclusion emphasizes the urgent need for effective antiviral agents and vaccines to combat the ongoing pandemic, acknowledging that commercial vaccine production may not begin before 2021.