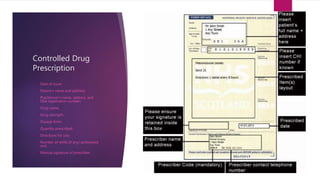
The document discusses the history and classification of controlled substances in the United States. It begins with a brief history of increasing drug regulation since the early 1900s, culminating in the 1970 Controlled Substances Act (CSA) which established five drug schedules based on a drug's medical use, abuse potential, and safety. The CSA contains laws governing the manufacture, distribution, and possession of controlled substances. The document then outlines the five drug schedules, providing examples of drugs in each schedule. It concludes by discussing prescribing, procurement, storage, dispensing, inventory control, and disposal of controlled substances in accordance with the CSA.