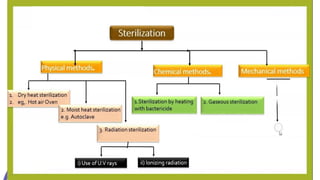
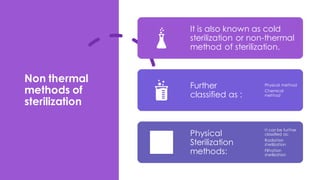
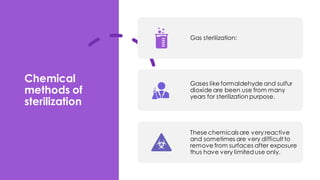
This document discusses control of microbial contamination in sterile and non-sterile pharmaceutical products. It describes sterilization methods like thermal sterilization using dry heat or moist heat, and non-thermal sterilization using radiation, filtration, or chemicals. It also discusses sources of contamination and control measures like environmental control, housekeeping, disinfecting surfaces, controlling air and personnel, automated processing, and concludes that sterilization requires constant effort to control microbial contamination.