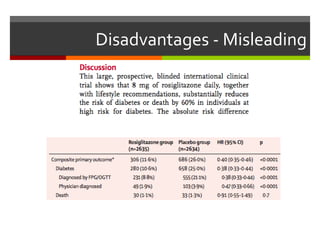
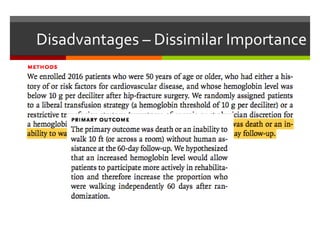
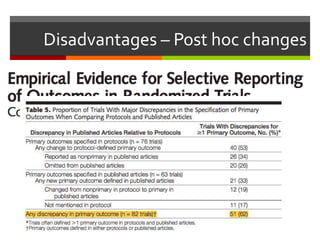
Composite endpoints combine multiple outcomes into one measure in clinical trials, offering advantages such as statistical efficiency and a broader evaluation of clinical benefits. However, they can be misleading if components have varying importance or if post hoc changes are made. Proper assessment of composite endpoints is critical to ensure components are of similar importance and frequency.