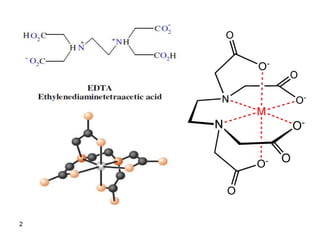
The document details the principles and applications of EDTA titrations, emphasizing its role as a chelating agent for complexometric titrations of divalent and polyvalent metal ions, with a stoichiometry typically of 1:1. It discusses the formation constants of EDTA complexes, the effect of pH on titration curves, and the use of indicators in these titrations, along with several practical examples and calculations. Overall, this document serves as a comprehensive guide on the methodologies and chemical dynamics involved in EDTA titrations.
![3
EDTA Equilibria
EDTA can be regarded as H4Y where in solution
we will have, in addition to H4Y, the following
species: H3Y-, H2Y2-, HY3-, and Y4-. The
amount of each species depends on the pH
of the solution where:
a4 = [Y4-]/CT where:
CT = [H4Y] + [H3Y-] + [H2Y2-] + [HY3-] + [Y4-]
The species Y4- is the ligand species in EDTA
titrations and thus should be looked at
carefully.](https://image.slidesharecdn.com/complexometric-titrationspart2-230417211617-7faa97e2/85/Complexometric-Titrations-part-2-ppt-3-320.jpg)

![5
The Formation Constant
Reaction of EDTA with a metal ion to form a
chelate is a simple reaction. For example, EDTA
reacts with Ca2+ ions to form a Ca-EDTA chelate
forming the basis for estimation of water
hardness. The reaction can be represented by
the following equation:
Ca2+ + Y4- = CaY2- kf = 5.0x1010
Kf = [CaY2-]/[Ca2+][Y4-]
The formation constant is very high and the
reaction between Ca2+ and Y4- can be considered
quantitative. Therefore, if equivalent amounts of
Ca2+ and Y4- were mixed together, an equivalent
amount of CaY2- will be formed.](https://image.slidesharecdn.com/complexometric-titrationspart2-230417211617-7faa97e2/85/Complexometric-Titrations-part-2-ppt-5-320.jpg)








![14
Example
Calculate the pCa of a solution at pH 10 after
addition of 100 mL of 0.10 M Ca2+ to 100 mL
of 0.10 M EDTA. a4 at pH 10 is 0.35. kf =
5.0x1010
Solution
Ca2+ + Y4- = CaY2-
mmol Ca2+ = 0.10 x 100 = 10
mmol EDTA = 0.10 x 100 = 10
mmol CaY2- = 10
[CaY2-] = 10/200 = 0.05 M
Therefore, Ca2+ will be produced from partial
dissociation of the complex](https://image.slidesharecdn.com/complexometric-titrationspart2-230417211617-7faa97e2/85/Complexometric-Titrations-part-2-ppt-14-320.jpg)
![15
Ca2+ + Y4- D CaY2-
CT = [H4Y] + [H3Y-] + [H2Y2-] + [HY3-] + [Y4-]
Kf = [CaY2-]/[Ca2+]a4CT
[Ca2+] = CT
5.0x1010 = 0.05/([Ca2+]2 x 0.35)
[Ca2+] = 1.7x10-6 M
pCa = 5.77
Using the same type of calculation we
are used to perform, one can write the
following:](https://image.slidesharecdn.com/complexometric-titrationspart2-230417211617-7faa97e2/85/Complexometric-Titrations-part-2-ppt-15-320.jpg)
![16
Kf = [CaY2-]/[Ca2+][Y4-]
5.0x1010 = (0.05 – x)/(x* a4 x)
assume that 0.05>>x
x = 1.7x10-6
Relative error = (1.7x10-6/0.05) x 100 = 3.4x10-3%
[Ca2+] = 1.7x10-6 M
pCa = 5.77](https://image.slidesharecdn.com/complexometric-titrationspart2-230417211617-7faa97e2/85/Complexometric-Titrations-part-2-ppt-16-320.jpg)


![19
Example
Find pCa in a 100 mL solution of 0.10 M Ca2+ at pH
10 after addition of 0, 25, 50, 100, 150, and 200
mL of 0.10 M EDTA. a4 at pH 10 is 0.35. kf =
5x1010
Solution
Again, we should remember that EDTA reactions
with metal ions are 1:1 reactions. Therefore, we
have:
Ca2+ + Y4- D CaY2- kf = 5.0x1010
1. After addition of 0 mL EDTA
[Ca2+] = 0.10 M pCa = 1.00](https://image.slidesharecdn.com/complexometric-titrationspart2-230417211617-7faa97e2/85/Complexometric-Titrations-part-2-ppt-19-320.jpg)
![20
2. After addition of 25 mL EDTA
Initial mmol Ca2+ = 0.10 x 100 = 10
mmol EDTA added = 0.10 x 25 = 2.5
mmol Ca2+ left = 10 – 2.5 = 7.5
[Ca2+]left = 7.5/125 = 0.06 M
In fact, this calcium concentration is the major source
of calcium in solution since the amount of calcium
coming from dissociation of the chelate is very
small, especially in presence of Ca2+ left in solution.
However, let us calculate the amount of calcium
released from the chelate:
mmol CaY2- formed = 2.5
[CaY2-] = 2.5/125 = 0.02 M](https://image.slidesharecdn.com/complexometric-titrationspart2-230417211617-7faa97e2/85/Complexometric-Titrations-part-2-ppt-20-320.jpg)
![21
Kf = [CaY2-]/[Ca2+][Y4-]
5x1010 = (0.02 – x)/((0.06 + x) * a4 x)
assume that 0.02>>x
x = 1.9x10-11
The assumption is valid even without verification.
[Ca2+] = 0.06 + 1.9x10-11 = 0.06 M
pCa = 1.22](https://image.slidesharecdn.com/complexometric-titrationspart2-230417211617-7faa97e2/85/Complexometric-Titrations-part-2-ppt-21-320.jpg)
![22
3. After addition of 50 mL EDTA
mmol EDTA added = 0.10 x 50 = 5.0
mmol Ca2+ left = 10 – 5.0 = 5.0
[Ca2+]left = 5.0/150 = 0.033 M
We will see by similar calculation as in step
above that the amount of Ca2+ coming from
dissociation of the chelate is exceedingly
small as compared to amount left. However,
for the sake of practice let us perform the
calculation:
mmol CaY2- formed = 5.0
[CaY2-] = 5.0/150 = 0.033 M](https://image.slidesharecdn.com/complexometric-titrationspart2-230417211617-7faa97e2/85/Complexometric-Titrations-part-2-ppt-22-320.jpg)
![23
Kf = [CaY2-]/[Ca2+][Y4-]
5x1010 = (0.033 – x)/((0.033 + x)* a4 x)
assume that 0.033>>x
x = 5.7x10-11
The assumption is valid even without verification.
[Ca2+] = 0.033+ 5.7x10-11 = 0.033 M
pCa = 1.48](https://image.slidesharecdn.com/complexometric-titrationspart2-230417211617-7faa97e2/85/Complexometric-Titrations-part-2-ppt-23-320.jpg)
![24
4. After addition of 100 mL EDTA
mmol EDTA added = 0.10 x 100 = 10
mmol Ca2+ left = 10 – 10 = 0
This is the equivalence point. The only source for Ca2+
is the dissociation of the Chelate
mmol CaY2- formed = 10
[CaY2-] = 10/200 = 0.05 M
Ca2+ + Y4- D CaY2- kf = 5.0x1010](https://image.slidesharecdn.com/complexometric-titrationspart2-230417211617-7faa97e2/85/Complexometric-Titrations-part-2-ppt-24-320.jpg)
![25
Kf = [CaY2-]/[Ca2+][Y4-]
5x105 = (0.05 – x)/(x* a4 x)
assume that 0.05>>x, x = 1.7x10-6
Relative error = (1.7x10-6/0.05) x 100 = 3.4x10-3%
[Ca2+] = 1.7x10-6 M, pCa = 5.77
5. After addition of 150 mL EDTA
mmol EDTA added = 0.10 x 150 = 15
mmol EDTA excess = 15 – 10 = 5.0
CT = 5.0/250 = 0.02 M
mmol CaY2- = 10
[CaY2-] = 10/250 = 0.04 M
Ca2+ + Y4- D CaY2- kf = 5.0x1010](https://image.slidesharecdn.com/complexometric-titrationspart2-230417211617-7faa97e2/85/Complexometric-Titrations-part-2-ppt-25-320.jpg)
![26
Kf = [CaY2-]/[Ca2+][Y4-]
5x1010 = (0.04 – x)/(x* a4(0.02 + x) )
assume that 0.02>>x
x = 1.1x10-10
The assumption is valid
[Ca2+] = 1.1x10-10 M
pCa = 9.95](https://image.slidesharecdn.com/complexometric-titrationspart2-230417211617-7faa97e2/85/Complexometric-Titrations-part-2-ppt-26-320.jpg)
![27
6. After addition of 200 mL EDTA
mmol EDTA added = 0.10 x 200 = 20
mmol EDTA excess = 20 – 10 = 10
CT = 10/300 = 0.033 M
mmol CaY2- = 10
[CaY2-] = 10/300 = 0.033 M
Ca2+ + Y4- D CaY2- kf = 5.0x1010](https://image.slidesharecdn.com/complexometric-titrationspart2-230417211617-7faa97e2/85/Complexometric-Titrations-part-2-ppt-27-320.jpg)
![28
Kf = [CaY2-]/[Ca2+][Y4-]
5x1010 = (0.033 – x)/(x* a4(0.033 + x) )
assume that 0.033>>x
x = 5.7x10-11
The assumption is undoubtedly valid
[Ca2+] = 5.7 x10-11 M
pCa = 10.24](https://image.slidesharecdn.com/complexometric-titrationspart2-230417211617-7faa97e2/85/Complexometric-Titrations-part-2-ppt-28-320.jpg)