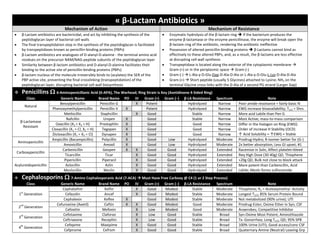
Compiled Antibiotic Table for study and review
- 1. « β-Lactam Antibiotics » Mechanism of Action Mechanism of Resistance β-Lactam antibiotics are bactericidal, and act by inhibiting the synthesis of the peptidoglycan layer of bacterial cell walls The final transpeptidation step in the synthesis of the peptidoglycan is facilitated by transpeptidases known as penicillin-binding proteins (PBPs) β-Lactam antibiotics are analogues of D-alanyl-D-alanine - the terminal amino acid residues on the precursor NAM/NAG-peptide subunits of the peptidoglycan layer Similarity between β-lactam antibiotics and D-alanyl-D-alanine facilitates their binding to the active site of penicillin-binding proteins (PBPs) β-lactam nucleus of the molecule irreversibly binds to (acylates) the SER of the PBP active site, preventing the final crosslinking (transpeptidation) of the peptidoglycan layer, disrupting bacterial cell wall biosynthesis Enzymatic hydrolysis of the β-lactam ring if the bacterium produces the enzyme β-lactamase or the enzyme penicillinase, the enzyme will break open the β-lactam ring of the antibiotic, rendering the antibiotic ineffective Possession of altered penicillin-binding proteins β-Lactams cannot bind as effectively to these altered PBPs, and, as a result, the β-lactams are less effective at disrupting cell wall synthesis Transpeptidase is located along the exterior of the cytoplasmic membrane Gram-(+) or in the periplasmic space Gram-(-) Gram-(-) L-Ala-γ-D-Glu-Dap-D-Ala-D-Ala or L-Ala-γ-D-Glu-L-Lys-D-Ala-D-Ala Gram-(+) Short peptide (usually 5 Glycines) attached to Lysine; NH2 on the terminal Glycine cross-links with the D-Ala of a second PG strand (Larger Gap) Penicillins 6-Aminopenicillanic Acid (6-APA), The Warhead; Ring Strain is Key (Azetidinone 4-Sided Ring) Class Generic Name Brand Name PO IV Gram (+) Gram (–) β-LA Resistance Spectrum Note Natural Benzylpenicillin Penicillin G X Potent Hydrolyzed Narrow Poor amide resonance = fairly basic N Phenoxymethylpenicillin Penicillin V X Potent Hydrolyzed Narrow EWG increase bioavailability; T1/2 = 5hrs β-Lactamase Resistant Methicillin Staphcillin X Good Stable Narrow More acid Labile than Pen G Nafcillin Unipen X Good Stable Narrow Most Active; mass-to-mass comparison Oxacillin (X1 = X2 = H) Prostaphlin X Good Good Narrow Differ in the Halogen on Ring (EWG) Order of increase H Stability (OCD) ↑ Acid Solubility = ↑EWG = Stable Cloxacillin (X1 = Cl, X2 = H) Tegopen X Good Good Narrow Dicloxacillin (X1 = X2 = Cl) Dynapen X Good Good Narrow Aminopenicillin Ampicillin (Bacampicillin) Polycillin X Good Low Hydrolyzed Moderate Prodrug-Hydro; R-isomer better for (G-) Amoxicillin Amoxil X Good Low Hydrolyzed Moderate 2x better absorption; Less GI upset; #1 Carboxypenicillin Carbenicillin Geopen X X Good Good Hydrolyzed Extended Racemize in Soln, Affect platelet=bleed Ticarcillin Ticar X Good Good Hydrolyzed Extended Req High Dose (30-40g) QD, Thiophene Acylureidopenicillin Pipericillin Piperacil X Good Good Hydrolyzed Extended +20g QD, Bulk not close to block attack Azlocillin Azlin X Good Good Hydrolyzed Extended More potent than Carbencillin, Acid Labile; Mezlo forms sulfonamide Mezlocillin Mezlin X Good Good Hydrolyzed Extended Cephalosporins 7-Amino Cephalosporanic Acid (7-ACA) Must Have Free Carboxy @ C4 (1 or 2 Step Process) Class Generic Name Brand Name PO IV Gram (+) Gram (–) β-LA Resistance Spectrum Note 1 st Generation Cephalothin Keflin X Good Modest Stable Moderate Thiophene; R2 = Acetoxymethyl -Activity Cefazolin Ancef X Good Modest Stable Moderate Longest T1/2; 85% Serum Protein Bound Cephalexin Keflex X Good Modest Stable Moderate Not metabolized (90% urine); UTI 2 nd Generation Cefuroxime (Axetil) Ceftin X X Good Modest Good Moderate Prodrug-Ester; Oxime Ether in Syn; CSF Cefoxitin Mefoxin X Low Modest Good Moderate Anaerobes; Competitive Inhibitor 3 rd Generation Cefotaxime Claforan X Low Good Stable Broad Syn-Oxime Most Potent; Aminothiazole Ceftriaxone Recephin X Low Good Stable Broad Tx: Gonorrhea; Long T1/2, QD; 95% SPB 4 th Generation Cefepime Maxipime X Good Good Stable Broad 100% Urine (UTI); Good access/conc CSF Cefpirome Cefrom X Good Good Stable Broad Quaternary Amine (Neutral) Leaving Grp
- 2. Monobactams β-Lactam not Fused with Another Ring Class Generic Name Brand Name PO IV Gram (+) Gram (–) β-LA Resistance Spectrum Note Sulfonated Sulfazecin Weak Weak Very Stable Weak Not used, C4 modification increase uses Aztreonam Azactam X Good Very Stable Narrow Tx: P. aeriginosa in Cystic Fibrosis Pts Carbapenems Suspsible to Metallo-β-Lactamases Class Generic Name Brand Name PO IV Gram (+) Gram (–) β-LA Resistance Spectrum DHP-1 Note Thienamycin Derivative Imepenem X Good Good Very Stable Very Broad Rapidly Hydrolyzed SE: Seizure; Not Abx, Reversible Meropenem Merrem X Low Good Stable Very Broad Slowly Hydrolyzed Antipseudo; Pene. CSF, Heart, Lung Ertapenem Invanz X Good Good Stable Very Broad Resistant QD dosing; Penetrate Porin Channel Doripenem Doribax X Good Good Stable Very Broad Slowly Hydrolyzed Includes Anaerobes, Antipseudo « β-Lactam Inhibitors » Mechanism of Action Type of β-Lactamases Avoidance – alter the structure of the penicillin so it is no longer a substrate for the β-lactamase Successful but decrease antibacterial activity Neutralization – inhibit the β-lactamase while simultaneously administering a penicillin that would otherwise be hydrolyzed The Serine β-Lactamases: Classes A, C & D Destroy the β-lactams antibiotics by a mechanism that is similar to how the β-lactams inactivate the PBP targets BUT, the acyl-enzyme complex is unstable easily hydrolyzed by H2O Some achieved Catalytic Perfection They catalyze as fast as they diffuse in Zinc-requiring β-lactamases (metallo-β-lactamases): Class B Catalyze the hydrolysis of S-D-lactoyl-glutathione to form glutathione and D- lactic acid and a competence protein that is essential for natural transformation and could be a transporter involved in DNA uptake. These proteins bind two zinc ions per molecule as cofactor In Gram-(+) bacteria that produce β-lactamases the enzymes are secreted from the cell or found along the exterior of the cytoplasmic membrane Hydrolyzed before reaching porous cell wall In Gram-(-) species the enzymes are found in the periplasmic space or bound to the exterior of the cytoplasmic membrane 2 nd line of defense if it makes it through the outer membrane Class 1 Example Oxapenam Inhibitor Antibiotic Used Brand Name PO IV Gram (+) Gram (–) Antibacterial Spectrum Note Clavulanate Amoxcillin Augmentin X X X Weak Broad Synergistic Effect coadmin with β-lactam; Extends life of PCN (Sacrificial Pawn) Ticarcillin Timentin X X X Weak Broad Sulbactam Ampicillin Unasyn X X X Weak Broad Potentiates activity of ampicillin/carbenicllin Tazobactam Pipericillin Zosyn X X X Weak Broad Synthetic; Triazole containing Class 2 Example Carbapenam Inhibitor Antibiotic Used Brand Name PO IV Gram (+) Gram (–) Antibacterial Spectrum Note Thienamycin Good Not used but a precursor to Carbapenems Cilastatin Imepenem Primaxin X None None Inhibits Renal Dehydropeptidase-1 (DHP-1)
- 3. « Peptide Antibiotics » General Mechanism of Action General Problems of Class Block cell wall biosynthesis by binding to peptidoglycan and its precursors Affects membrane permeability by forming pores (leaky) and alter functions Bactericidal and Inhibits protein biosynthesis Acid labile, Short duration, Enzymatically Hydrolyzed, Antigenic, Spendy, Toxicity Membrane is usually a problem cause its hard to differentiates from bacteria and host cells It attacks everything Peptides Every Organism Produce some Type of Protective Peptide (Multi=Defensin; Uni=NRPS) Mechanism of Action Mechanism of Resistance Blocks the diphosphatase that converts undecalprenyldiphosphate to undecaprenylphosphate Inhibits Translocation (Stalls assembly line) Inhibits recycling of the undecalprenyldiphophate lipid carrier (Building Blocks) Requires Zinc and Magnesium for activity Prevents Diphophatase from accessing the substrate Increased de novo synthesis of C-isoprenyl phosphates (IP) which is a carriers during synthesis of the repeat subunits of peptidoglycan Substrate-Binding Inhibitor Inhibits reaction by blocking substrate not enzyme Thiazoline Segment is Essential for Bioactivity Class Generic Name Brand Name PO IV Gram (+) Gram (–) Side Effects Note Cyclic Peptides Bacitracin Bacitracin Topical X Nephrotoxic Neosporin (Broad) = Bacitracin (+), Neomycin (+/-), Polymyxin (-) Affect Cell Membranes Polymyxin/Colistin Xignis Top/IV X Neuro/Nephro Tx: Sepsis (Endotoxin) and Septic Shock; Poor selective toxicity MoA Complex Lipid A (LPS); affect permeability/disorganize MoR Target Modification by inducible electrostatic repulsion Gramicidin Topical X X Hemolysis Alternating D/L AA forms helix; Not charged, N=Formyl; C=Ethanol MoA Form pores in G+ cytoplasmic membrane; Requires 2 helices to span membrane; Permits cations to escape, Depolarizing (K/Na) Daptomycin Cubicin X X Low/GI Prob Tx: SSSI and Infective Endocarditis/Bacteremia; MSSA, MRSA, VRE MoA Ca 2+ Dependent Abx; Conformation to pump, Depolarize MoR None are known, but can develop; No cross-resistance Affect Protein Synthesis Quinu/Dalfo-pristin (Streptogramin) Synercid X X Pain Inject Site Tx: VRE/MRSA; -static alone, synergistic = -cidal; Inhibit CYP3A4 MoA 50S Bacterial Ribosomal; Prevent protein elongation MoR Drug Modifying Enzyme and Target Modification Glycopeptides Family of Highly Modified Rigid Peptides with One or More Aminosugar Mechanism of Action Mechanism of Resistance Inhibits both Transglycosylation AND Transpeptidation (Blocks PG elongation) Forms a strong non-covalent complex with D-Ala-D-Ala terminus of Lipid II and PG Prevents binding of the substrate to TPase/TGase causing steric bulk hindering access to enzyme Affects only the outer surface of the cells Inhibit the synthesis of cell walls in susceptible microbes by inhibiting PG synthesis. They bind to the AA within the cell wall preventing the addition of new units to the PG. They bind to acyl-D-alanyl-D-alanine in peptidoglycan. VanA: High-level resistance to both teicoplanin and vancomycin. Resistance is induced by presence of the antibiotics. Resistance genes are transmitted between bacteria on a transposon. VanA is the enzyme (a ligase) that forms the D-Ala-D-Lac VanB: Resistant to vancomycin but susceptible to teicoplanin. Resistance is only induced by vancomycin. VanB is a ligase that forms the D-Ala-D-Lac VanC: Intrinsic resistance to vancomycin but not teicoplanin. Constitutive expression of VanC gene that encodes VanC, a D-Ala-D-Ser ligase Class Generic Name Brand Name PO IV Gram (+) Gram (–) Side Effects Note Tricyclic Heptapeptide Vancomycin Vancocin X X X Nephro/Oto Toxic Oral Tx: C.Diff; Usually -cidal/static; Synergy with AG 1 st Generation Lipoglycopeptides Teicoplanin Targocid X X Hypersensitivity 2 nd MoA Direct Inhibition of Transglycosylase Telavancin Vibativ X X Hypersensitivity Antibacterial Inhibit PG synthesis, Permeability 2 nd Generation Lipglycopeptides Dalbavancin Zeven X X Still in Trials Active vs VanB not VanA VRE; Greater potency; Qwk Oritavancin X Still in Trials Active vs both VanA/VanB VRE; Strong –cidal; QD
- 4. « Aminoglycoside Antibiotics » Mechanism of Action Mechanism of Resistance Transport AGs alter the outer membrane of (G-) bacteria allowing penetration through membrane and can pass through porins AGs are actively transported by an O2–dependent transport system (Low pH and anaerobic inhibit transport) Cellular Target Bind to the16S rRNA component of the 30S ribosomal subunit “irreversibly” inhibit protein biosynthesis = Bactericidal Interaction of AGs with the 16S rRNA of the 30S ribosomal subunit Numerous contacts are made mostly with nucleic acids NOT proteins Cause misreading of mRNA = Truncated proteins or proteins with the wrong amino acid sequence that do not fold correctly = Inhibit initiation of protein synthesis Cause dissociation of polysomes into non-functional monosomes Blocking the initiation of protein synthesis Initiation step (need many things to come together @ 5’- 3’ end of mRNA) 30s, 50s, Proteins, mRNA, fmet (formyl) Ribosome attach via 5’ Streptomycin binds, stalling translation hindering movement down the mRNA; Lost of fidelity Decrease Drug Uptake or Accumulation by altering porin channels in outer membrane Cannot be acquired, usually developed Active efflux (extrusion of toxic substances and antibiotics outside the cell) Can be acquired Alter ability of drug to cross the cytoplasmic membrane Results from changes in membrane proteins and alterations in regulation of genes in anaerobic respiratory pathway Energy-dependent process tied into ETC and cell respiration O2-Dependent Process This is why AGs NOT active against anaerobes Three types of Aminoglycoside Modifying Enzymes (AME) Bifunctional AG O-Phosphotransferases (APH) = Kinase; Any OH grp can be modified AG O-Nucleotidyltransferases (ANT) = eg. AMP, Causes steric bulk AG N-Acetyltransferases (AAC) = Any NH2 can be acetylated Streptomycin = 1 st AG Discovered Site of Action for AG on Bacterial Ribosomes affecting Protein Biosynthesis (Mycin=Streptomyces; Micin=Micromonospora) Class Generic Name Brand Name PO IV Gram (+) Gram (–) Treatment Note Class Properties Gentamycin Family Gentamicin Garamycin Top/IV X P. aeruginosa; Burn, CF Coadmin w/ β-Lactam = syngery Basic/charged at neutral pH Highly H2O Soluble Pretty toxic: Nephro/Ototoxic Netilmicin Netromycin X X Pink Eye Ethyl grp reduce inact. by AME Kanamycin Family Kanamycin Kantrex X X X Dysentery; MDR-TB Mixture of Kanamycin A Amikacin Amikin X X Gent/Tobr Resist; Myco 50% potent, less prone by AME Tobramycin Nebcin X X P. aerug, not Myco (TB) Use with antipseudo β-Lactam Neomycin Family Neomycin Mycifradin PO/Top/IV X X Burns Most nephrotoxic Paromomycin Humatin X X X Ameobic Dysentery Tx: Leshmaniasis (Parasidic-Fly) END OF MODULE 5 Bacteria Miscellaneous Information on Gram Stains Bacterium Name Gram (+) Gram (–) Bacterium Name Gram (+) Gram (–) Staphylococcus aureus MSSA, MRSA X Streptomyces X Pseudomonas aeruginosa X Enterobacteriaceae X Mycobacterium tuberculosis Weak Acinetobacter X Escherichia coli X Haemophilus influenzae X Enterococcus VRE X Neisseria gonorrhoeae X Streptococcus pneumoniae X Actinomycetes X Clostridium difficile X Coagulase Negative Staphylococci (CoNS) X
- 5. « Tetracycline Antibiotics » Mechanism of Action Mechanism of Resistance Inhibit protein synthesis by binding to the 16S rRNA of the 30S ribosomal subunit 6 TC binding sites on 30S Ribosome Block binding of the aminoacyl-tRNA to the ribosome Prevents elongation of peptide chain Reversible and bacteriostatic Selective toxicity results from … (Seldomly used due to toxicity) Poor penetration of mammalian cells and low affinity for mammalian ribosomes (80S ribosome) will disrupt mitochondria protein synthesis (70S) Some bacteria will concentrate these drugs in the cell Binding of TC to 16S rRNA of 30S Ribo Mg bridges drug-target like Bacitracin Pi-Pi stacking interaction with D-ring with C1054 No effects on efflux pumps…? Enzymatic Inactivation – Rare, Tet X minooxygenase put OH on TC ↓ activity NOT in any pathogen, 1 example of O-Acetyl Transferase Target Modification – Rare, Cl058G mutation ↓ Binding Energy-Dependent Efflux – ATP-required efflux pump actively transport drug out of cell before reaching effective (60%) concentration and protein synthesis halted Ribosomal Protection Proteins (RPP) – Found in G+/- species and some mycobacteria, on a chromosome or on a plasmid; resistance only at low TC conc. Relates to elongation factors (GTPases) required in protein synthesis EF-Tu & EF-G Tet (O) and Tet (M) bind to ribosome in GTP-dependent manner and induce dissociation of TC/Ribo complex Protein Synthesis continues RPP free the ribo from TC bound at Tet-1 site = free drug can still bind to other sites; 6 Tet sites on a ribosome reason its effective in low TC Conc Protein Synthesis Inhibitors Effective against many organisms resistant to agents acting on the cell wall Class Generic Name Brand Name PO IV G(+) G(–) Treatment Note Old Generation Chlortetracycline Aureomycin Topical X X Gold color; Misused/Overused; Precursor for other TC; Photosensitivity C7 Tetracycline Achromycin V X X X H. Pylori Combo w/ Bismuth & Metronidazole; High Plasma conc, Long Duration Oxytetracycline Terramycin X X X Note C6 difference OH and CH3 Combined to form Doxycycline via [H] Methacycline Rondomycin X X Note C6 difference CH2 double bond Doxycycline Vibramycin X X X X Lack C6 OH; Decrease Ca2 Binding; Retains Mg Binding Minocycline Minocin X X X X Dimethylamine C7; No C6 OH; Best absorbed; Longest T1/2 New Gen. Tigecycline Tygacil X X X MRSA & Anaerobe Add’l Contact w/ 16s rRNA; Not efflux pump; Active Tet-res bac; 5x bind aff. « Tetracycline Extra Information » General Features SAR C6 OH can degrade via acid/base C4 center can epimerize in weak acid ↓active Coord (Chelates) w/ Metal Ions (Mg, Ca, Fe, Al) required for entry to G(-) cells Mg required to pass through porinrelease periplasmicapo-formchelates a-Stereochemical Config important for activity (AC4a) Removal of dimethylamine reduces activity (AC4) Keto-Enol system in proximity to D-Ring is important for activity (AC1/CC11) Basic Nitrogen of glycyl unit is essential (DC9) Must retain linear fused ring system must be six-membered and purely carbocyclic Targets for chelation with Divalent cations (Both; AC12a) Contraindications and Side Effects If taken with dairy products, many of these agents are poorly absorbed Ca and Mg forms an insoluble complex CI: Anti-acid Combo of broad spectrum of activity and poor absorption lead to superinfections Candida, Clostridum Opportunistic Infections Tetracycline/Ca2+ complex can be deposited in teeth and bone during gestation and early childhood Not for pregnant women to use Small percentage of patients develop photosensitivity (UV Absorptive=C7 Halogen) pKa1 pKa2 Three ionizable groups and exist as zwitterions at neutral pH Amphoteric = A salt react with either acid or base pKa3
- 6. « Macrolide Antibiotics » Mechanism of Action Mechanism of Resistance Reversibly bind to the 50S bacterial ribosomal subunit Specifically 23S rRNA Inhibit translocation of the growing protein chain (Elongation) A-P Site move Selective toxicity is achieved because these agents do not bind to mammalian ribosomes (80S at therapeutic concentration Usually bacteriostatic Stops replication Drug binds to polypeptide exit tunnel (Blocking) of bacterial 50S ribosomal subunit Intrinsic resistance in Gram-(-) organisms is probably due to impermeability of outer membrane to the hydrophobic macrolides Acquired resistance involves three mechanisms Target Modification resistance mechanism found in the antibiotic producer Posttranscriptional methylation of 23S rRNA (A2058) Cross Resistance to Macrolides, Lincosamides and Streptogramin B-type MLS resistance phenotype Also Resistance to azalides Erythromycin Resistance Methylase (erm) genes Erm A most common Can be chromosomal or plasmid encodes (Transmittable) This mechanism accounts for almost all resistant isolates in clinical practice Drug Inactivation (Inactive Kinase) Erythromycin esterases type I and II hydrolyzes the lactone to a linear chain Macrolide 2’-phosphotransferase Phosphorylates the 2’-OH of desosamine Analogous to AG PPT Active efflux ATP- dependent drug efflux pump reported for S. epidermidis General Information Natural antibiotics (Polyketide) Characerized by Large Cyclic Esters (Lactone):14 Cladinose and Desosamine, may be attached Ring is highly functionalized (Me/OH) Role in acid degradation in GI disturbance Complex stereochemistry; Aminosugar, Neutral sugar, Ketone Spectrum is similar to Penicillins (Gram +) and safe but with limited Hepatoxicity Can be used when patient is sensitive to pencillins Unstable in pH ≤4 Weak bases (Salt formulation); intramolecular rearrangement Protein Synthesis Inhibitors Effective against many organisms resistant to agents acting on the cell wall Class Generic Name Brand Name PO IV G(+) G(–) Treatment Note 1 st Generation (Erythromycin) Erythromycin A X X X Ear Infections Acid Labile; Very Bitter; Salts and Ester prodrug ↑ Bioavailability and ↓ Bitter Stearate Ethril X X Stearate Salt 18C saturated FA; Free base liberated in alkaline duodenum Ethyl Succinate Pediamycin X X 2’ OH of Desosamine is esterified with ES, absorbed as ester then hydrolyzed Estolate Ilosone X X Same as above w/ Propionate Ester; Lauryl Sulfate Salt (12C sat); Acid Stable 2 nd Generation (Derived from Erythromycin) Clarithromycin Biaxin X X X Disseminate MAC MAC Birds/Humans; Acid Stable (C6); Legionella (G-); Rapid 1 st Pass (14-OH) Azithromycin Zithromax X X X Gonrrhea Azalide (N); Acid Stable (No Ketone); High Tissue Conc 2 binding site on 23S Roxithromycin Rulid X X X Soft Tissue Infect Methoxyethoxymethyl eher of EryA Oxime; Better in vivo; Absp/Dist superior Dirithromycin Dynabac Not In US Market Prodrug of Erythromycyclamine; Product of reduce EryA oxime, Imp Acid Stable Telithromycin Ketek X X X Com-Acq-Pneum 1 st Ketolide No induce resistance (Mask); QD dosing, Acid Stable; Mya-Gravis Others Affecting Protein Biosynthesis Class Generic Name Brand Name PO IV G(+) G(–) Treatment Note Lincosamides Clindamycin Cleocin X X Staph Infection in Bones and Joints Usually not DOC; Active metabolites (N-demethyl) and Sulfoxide; Can lead to Fatal form of Colitis; Superinfection resistant producing Clostridium Lincomycin Lincocin X X MoASame as macrolides 23S rRNA of 50S ribosome MoRSame as macrolides (ErmA MLS Phenotype); Drug medication O-Nucleotidyl Xase Oxazolidinones Linezolid Zyvox X X X X MRSA / VRE Reserved Uses; Inhibitors MAO reversible nonselective; Mainly Gram Positive MoAOverall Distrupt protein syn, Bind 23S rRNA n ear interfeace w/ 30S; Block 70S MoRPoint Mutation 23S rRNA; G2576T changes to Uracil No binding; No X-Resist Old Drug Chloramphenicol Chloromycetin X X X X Empirical Tx Overused; Penetrate BBB; Active vs anaerobes; BONE MARROW TOX, ↑aff 70S MoABlock binding of Aminoacyl-tRNA, Inhibit peptide bond; Compete Macrolide Site MoRX-Resist, Ery/Linco/Strep Methylation (MLS Resist); Alteration acetylate CAT Protein Inhibitor Retapmulin Altabax Topical X Imeptigo (MSSA) Affect the protein;-static/↑*-cidal]; Fungus; Used in Vet (Swine); No X-Resist MoAAct at Ribosomal Protein L3; Block P site, Inhibit Peptidyl Transfer; Prevent active MoRMutation at Ribosomal L3; Presences of Efflux; Hydrolase will destroy Ester
- 7. « Fluoroquinolones Antibiotics » Mechanism of Action Mechanism of Resistance Disrupt DNA Replication/function (Folding and wadding) Usually Bactericidal Targets TWO topoisomerases (II-Gyrase and IV) = Controls coiling, topological DNA Gyrase Relaxes AND supercoil DNA ATP-dependent process Topo IV is a DECATENATING (Unties) enzyme Unknot bring up DNA links Some FQ inhibits both of these, some just one Gyrase and Topo IV bind to double stranded DNA and they break both strands FQs bind and trap the intermediate in which the topoisomerase subunits are covalently bound to broken DNA. Bacteriostatic? Release of fragmented chromosome correlates with cell death generate ROS Quinolones only recognize and bind the topoisomerase-DNA complex Selective toxicity: mammalian cells don’t have DNA gyrase and quinolones have low affinity of for mammalian topoisomerase II Mutations in gyrase and topo IV genes – nearly always chromosomal mutation Arise from point mutation in gyrA, gene that codes for DNA gyrase subunit A parC and parE mutants are resistant, but less common Genes that encode the subunits of topoisomerase IV Decreased intracellular accumulation of the drug Modifications of membrane proteins Efflux Pumps In some S. aureus, the recG gene product can repair fluoroquinolone damage Plasmid-borne fluoroquinolone resistance (Transferable) Qnr proteins Interfere with quinolone binding to gyrase and topoisomerase IV Proposed to recognize gyrase/topo IV and alter DNA binding properties Fluoroquinoline-modifying enzymes An aminoglycoside acetyltransferase (AAC(6’)-Ib) has mutated to modify cipro- and norfloxacin Bifunctional – it will still modify AGs Efflux pumps The plasmid-encoded pump QepA can increase MIC by 10-fold Side Effects and Toxicity Affects Cartilage, rupture tendon/Tendonitis, OT Prolongation, Photosensitivity Inhibitors of Nucleic Acid Metabolism and Function FQs Cross-Resistance is often seen Class Generic Name Brand Name PO IV G(+) G(–) Treatment Note 1 st Gen. Nalidixic Acid Neggram X X Uncomplicated UTI Non-Fluroinated, Resistance widespread, Limited spectrum; Strong acid 2 nd Generation Nofloxacin Noroxin X X X UTI / Others Fluroquinolone; 100x more potent and broader spectrum; Widely used Ciprofloxacin Cipro X X X UTI / Others Better absorption; Potent vs Pseudomonas; Safe; Bacillus Anthracis Oflxacin Floxin X X X Mycobacteria; Leprosy / TB S-isomer most active; 8-125x difference in potency; High CSF concentration Levofloxacin Levaquin X X X X 3 rd Generation Sparfloxacin Zagam X X X CAP Potent vs strep/anaerobes; Difluro; T1/2=18hrs; Low photosensitivity Gatifloxacin Tequin X X X X Withdrawn Cause blood sugar fluctuation (Stim insulin secretion); Kidney/Liver Failure 4 th Generation Moxifloxacin Avelox X X X X Tuberculosis Bactericidal; Lower resistances; Not metabolized by P450; Inhibit Topo II/IV Gemifloxacin Factive X X X CAP/AECB Potent inhibitor of TOPO IV and GYRASE in vitro; in vivo Gyrase perferred « FQs Structure Activity Relationships » Position 2 should be unsubstituted N-1 must have a small alkyl group attached (Methyl, Ethyl, Cyclopropyl) N can replace C at position 8 without loss of activity (Nalidixic Acid) Groups at C-5 and position 8 can affect photosensitivity side effects Fluorine at C-6 increases activity Substituents at C-7 often increase activity Piperazine extends spectrum to include Ps. Aeruginosa Type of substituent can affect selective toxicity
- 8. « Antifolate Agents » Mechanism of Action Mechanism of Resistance Coenzymes for biological processes Synthesis of thymidine and purines and AA Specifically – sulfonamides are competitive inhibitors of dihydropteroate synthase Resemblance to p-aminobenzoic acid (PABA) Serve as inhibitor OR alternate substrates for DHPS Replace PABA in the condensation reaction catalyzed by DHPS – the result is a dihydropteroate derivative that cannot be further transformed to tetrahydrofolate Inhibition of DHPS causes bacteriostasis Selective Toxicity It is a vitamin required in mammalian diets mammals do not synthesize folate Bacteria cannot use folate from mammalian diet to replace depleted levels Because bacteria have a de novo biosynthetic pathway for folate most have not developed transport systems for importing folate from the environment. Sulfonamides – major limitation to their use Organism overproduces PABA (Mutational) Acquired resistance from a plasmid-encoded DHPS that binds PABA normally but has very low affinity for sulfonamides Alterations that decreased permeability of the cell ↓Access (Mut Dev) Co-trimoxazole – less frequent but still observed Over 12 plasmid-borne genes expressing resistant DHFR variations are known examples of a chromosomal DHFR with lowered trimethoprim affinity Also find elevated DHPS and/or DHFR levels in some resistant bacteria (↑*Target+) SAR / General Features Sulfonamide NH is acidic Allowing it to tightly bind (pKa 5-10, useful at 6-7) High pKa can cause crystallizein kidney (pH 6); Left Aniline NH2 is essential, R1=H Sulfonamide Inhibition of Folic Acid Biosynthesis Inhibitors of Bacterial Cell Metabolism Class Generic Name Brand Name PO IV G(+) G(–) Treatment Note Prontosil Sulfisoxazole Gantrisin X X X UTI Most Acidic pKa=5; R2=Dimethylisoxazole; Bitter; Acetyl=Hydro in GI; Pediazole Sulfamethoxazole Gantanol X X X UTI pKa=6 Long T1/2~8hrs; Combine w/ TRIMETHOPRIM synergy Septra/Bactrim Others No real Class or MoA Generic Name Brand Name PO IV G(+) G(–) Treatment Side Effects Note Phosphomycin Monurol X X UTI No X-Resist not SE Phosphonate; Bactericidal vs E.coil/E.faecium; Static vs others species; Salt MoA Inhibits MurA (UDP-GlcNAc enoylpyruval transferase) – catalyzes 1st committed step in cell wall biosynthesis covalently binds to Cys115 in the MurA active site MoR fosA=Mn2+dept metalloglutathione transferase & fosB =catalyzes cysteine and Mg2+-dept & fosX=Mg2+-dept fosfomycin-specific epoxide hydrolase Mupirocin Bactroban Topical X Skin Infection Mimics Ile(Isoleucine) Binds to ATP binding site, Bacteriostatic; -cidal=low pH MoA Inhibits Protein synthesis via Bacterial Isoleucine tRNA (IleRS) blocking MoR Low-level=Nontransferable due to chromosomal mutation; High=insens Novobiocin Albamycin Topical X MRSA AminocoumarinWarfarin like could possibly replace it in future; Polyketide MoA Inhibits DNA Gyrase (Different from FQ, greater affinity for it); Specifically inhibit ATP Hydrolysis in GyrB No energy required for inducing supercoiling Metronidazole Flagyl X X X X Bacterial Vaginosis Urine Red/Brown, Cancer, Select Tox Tx: Paraistic infections Trichomoniasis and GI ; Access GI, Bactericidal, Active against Obligate anaerobic bugs, Antiprotozoan Tinidazole Tindamax X X X X MoA Nitro Group goes 1e- reduction; PFOR reduces ferredoxin; DNA/Protein damage MoR Resist to plasmid-borne gene encodes Nitroductase Amino=inactive Fosmidomycin Malaria In clinical Trials; Can be antibacterial; Phosphonate structure MoA Inhibits Isoprenoid biosynthesis (Via DXR in non-mevalonate pathway AKA MEP synthase), prevent formation of Sterols, Ubiq, transporter, etc HMG-CoA pathway
- 9. « Anti-Tubercular Agents » Tuberculosis Information Tuberculosis Resistance Contagious airborne disease caused by Mycobacterium tuberculosis (Mtb); 1-3 cells Typically affects lungs (Spread via human to human contacts (Seem on X-Ray) Mtb=Latent TB (DormantNon-Replicating), Hard to treat Complex outer membrane (a barrier that is denseWax-like) Very slow growing (15-18hrs) Long Therapy; Longer for resistant cases Reducing the spread of TB Direct Observe Therapy Short course (DOTS) Multidrug Resistant Mtb (MDR-TB) = Resistant to BOTH Isoniazid and Rifampin Add 2 nd Line injectable AG or Tuberactinomycin, and FQ Extensively Drug Resistant Mtb (XDR-TB) = Resistant to Isoniazid AND Rifampin PLUS FQ AND at least an injectable 2 nd Class Generic Name Brand Name PO IV G(+) G(–) Side Effects/Tx Note 1 st Line Isoniazid Nydrazid X X Hepatotox (N-Ace) Static vs Resting Cidal vs Dividing; Very Selective(Host no target); Low Tox MoA Targets the InhA in mycolic acid synthesisKatG Ox N-NRadical+NADH=AdductInhibits InhA MoRLoss of Permiability (↓access); Mutation in InhA (target alter) 1 st Line (Rifamycins Derivative) Rifampin Rifadin X X X Tx: TB and MRSA Active vs Dividing/Semidormant-sterile; Moisture sensitive-Hydrozone; CNS Rifapentine Priftin X X GINausea; Discoloration Body Fluid (Orange/Pink) Cyclopentyl Derivative; Less freq dosing All Rifamycins Strong P450 inducers; ↑Protease Inhibitor Metabolism; Bactericidal Rifabutin Mycobutin X X Tx: MAC; Less P450 actions; Prophylactic Rifaximin Xifaxan X X Tx: Traveler Diarrhea; <1% orally absorbed MoA Inhibits bacterial DNA-dependent RNA polymerase (β-Subunit)Blocks Elongation, prevent gene expression; ALLOSTERIC inhibitor; Chelates Zn/Mg; No effect on mam 1 st Line Ethambutol Myambutol X X Visual Acuity, Color Static vs Dividing; Synergistic w/ Rifampin; (+)Isomer potent; Fish tank/hand MoA Inhibits Mtb formation of cell wall arabinan; Interfere w/ arabinose acceptor MoRResults from acquisition of genes of Emb proteins (Dev Mut) Pyrazinamide Aldinamide X X Gout Good vs Dormant/Semidormant; Acidic Environment; FA Synthase1/activate 2 nd Line (AGs) Streptomycin Streptomycin X X Renal, Neural (Oto) Toxicity Bactericidal Kill TB outside of macrophages (Limited window of attack); Resistance due to Phosphorylation (APH); No effect vs MAC Kanamycin Kantrex X X X Amikacin Amikin X X 2 nd Line Ethionamide Trecator SC X X X GI Upset / NV Less potent, well distributed; Req Oxidative Activation (KatG) ethA gene Cycloserine Seromycin X X X CNS Toxicity MoA Block conversion of L-Ala to D-Ala (Inhibits Alanine Racemase); No X Aminosalicyclic Acid PAS Parasal X X X GI Upset (Acid) MoAAntifolat; Competitive inhibitor of Mycobacterial DHPS; Spec; Static Capreomycin Capastat X X X Renal, CNVIII Toxic Tuberactinomycin Abx; Highly basic, AG like SE (Oto); Resist=Lost of function MoA Interferes w/ Initiation of tRNA(Fidelity) and Elongation16S rRNA by AG w/ contacts 23S rRNA MoRDrug Mod (Ace/Pho); Target Mod (rRNA OMT) 2 nd Line (FQs) Levofloxacin Levaquin X X X X Tx: Mycobacteria; TB S-isomer most active; 8-125x difference in potency; High CSF concentration Moxifloxacin Avelox X X X X Bactericidal; Lower resistances; Not metabolized by P450; Inhibit Topo II/IV Experimental Agents Nitroimidazopyran PA-824 Under Development Affects Lipid/Protein Synthesis; Block late state of mycolic acids; Cidal both Diarylquinoline R207910 Long T1/2; Comb w/ INH, RIF, PZA; No X-resist; atpE geneProton Pumps « Anti-Fungal Agents » Amphotericin Mechanism of Action General Anti-Fungal information Amphotericin B complexes with ergosterol in the fungal membranes to form pores Permitting K+ and Mg2+ to escape Shuts down protein and DNA Synthesis Ergosterol is unique to fungi (Selective Toxicity) Ergosterol is similar enough to cholesterol structure that selectivity is not complete Results in toxicity, especially in the kidney Takes 8 AmpB molecule + 16 sterol to form HALF a poreneeds 2 HALVES for pore Fungi are eukaryotic=more complex; harder to treat Abx has no effect on fungi Share host features ie: Protein and Nucleic Acid synthesis (Drug blocks) Ergosterol Synthesis Azoles, Allylamines, Morpholines NA Synthesis/Functions 5-Fluorocytosine • Chitin Synthesis Nikkomycin Spindle Distruption/Antimitosis Griseofulvin • Protein Synthesis Sordarins Membrane Distruption Polyenes • Glucan Synthesis Echinochandins
- 10. AmphotericinsAgents Taken Systemically to Treat Systemic Mycoses (Fungal Infections) …Anti-Fungal Continues Class Generic Name Brand Name PO IV Cidal Treatment Side Effects Note Amphotericin B Fungizone X Yes Crytococcus Gattii Kidney Damage Gold Standard; Dose dependent; Shake n’ Bake; Amphoterrible Lipid Formulation Lipid Complex Abelcet X Yes Not 1 st line; Treat Invasive fungal infections ABLC; Ribbon-like configuration; Decrease free drug = ↓ Toxicity Colloidal Dispersion Amphocil X Yes ABCD; Disk-like (Cholesteryl Sulfate); ↑ incidence of Hypoxia/Chills Liposomal Ambisome X Yes Tx: Crytococcus LAMB; True Liposome (Spherical); ↑Conc in plasma=long duration Enchinocandins Affects the Exterior of Cells Cell Walls Class Generic Name Brand Name PO IV Cidal Treatment Side Effects Note Lipopeptide Antifungals Caspofungin Cancidas X Yes Invas.Aspergillosis Minimal Tx: Candida/Pneumocystis; Active vs Azole-Resist Strain; Acetylation MoA Inhibits 1,3-β-Glycan Synthase = Block assembly of fungal cell wall MoR Rare; Subsitution in Fks1p Subunit of 1,3-β-Glycan Synthase ↓susceptibility Micafungin Mycamine X Candidiasis Broad Spectrum; Prophylactic uses w/ AIDS pts; Active Azole-Resist Anidulafungin Eraxis X Candidiasis Tx: esophageal/candidemia/peritonitis; Lipophilic; Terphenyl Tail Flucytosine (5-FC) Ancobon X Usually in combo w/ Amphotericin B; Synthetic; Requires Activation MoA Interferes w/ Fungal Protein/DNA Synthesis (5-FCF-FU + FdUMP (Phos) dUMP + TS = dTMP & FdUMP blocks TS); 5-FC FUMP 5-FU = No Protein Azoles Anti-Fungals Mostly Triazoles or Imidazoles No Antibacterial Actions (Target Not Present) and Concentration Dependent at Site of Infection Mechanism of Action Mechanism of Resistance Overall= Disrupt Ergosterol SynthesisSpecifically=Inhibit Sterol C14a-demethylase Disruption=Lack of ergosterol (Downstream) for membrane function and fluidity Results in a build-up of C14-methylated sterols (Toxic=Ignosterol) that get into the cell membrane and lead to faulty function and leakage of cell contents Ianosterol Zymosterol via C-14 DeMEase via CYP51A1 (Oxidative) block by Azoles Reduced intracellular accumulation of drugs, due to either decreased uptake or increased efflux Altered (Mut) sterol C-14 demethylase or other ergosterol biosynthetic enzymes Amplification of genes encoding for target enzymes ↑Target Concentration All been identified in isolates of Candida obtained from pts failing azole therapy Class Generic Name Brand Name PO IV Cidal Treatment Side Effects Note Imidazole Ketoconazole Nizoral X Terato, Steroid P450 Inhibitor=Steroid Imbalance; Toxic=N-deactylate; Best low pH Triazole Fluconazole Diflucan X X Cryto Meningitis Better than Keto Tx: Oral/Esoph Candida & Pulmonary Cryto; Good Absorp/Dist+CSF Dubbed 2 nd Keto Itraconazole Sporanox X X Fungal Infection Better; Liver Fail 1 st DOC; Case of liver failure and ↓Cardiac Contraction Force (Rare) New Generation Triazoles Voriconazole Vfend X X Invas.Aspergillosis Visual/↑Liver Enz Outperformed AmpB in trials; 30% pts=Visual Disturbance (30min) Posaconazole Noxafil X Broader Spectrum and Better activity than Itraconazole Ravuconazole Phase 3 Trials Long T1/2 OD Dosing; Similar to Fluconazole but with Thiazole Agents Taken Systemically to Treat Non-Systemic Mycoses (Fungal Infections) Class Generic Name Brand Name PO IV Cidal Treatment Side Effects Note Older Agents Griseofulvin Fulvacin X No Hair/Nail Infection HA, Rash, GI Pain Fungistatic; Poor oral bioavailbility (Micronized increase it) MoA Inhibits fungal mitosis by preventing separation of chromosomes Blocks normal microtubule functions (Selective Toxicity unclear) Terbinafine Lamisil PO/Top Warts Liver Failure Allyl Amine; Concentrates in Stratum Corneum MoA Inhibits Esgosterol Biosynthesis Targets Squalene-2,3-Epoxidase (Don’t make epoxide); Squalene (Epoxidase) Epoxide Butenafine Mentax Topical Yes Dermatophytes Exerts Anti-Inflammatory in vivo; ↑Conc remains for prolong time Thiocarbamates Tolnaftate Tinactin Topical Ath. Foot/Jock Itch Allyl Amine; Inhibits squalene 2,3-epoxidase Naftifine Naftin Topical Jock Itch/Ringworm Allyl Amine New Agents Amorolfine Loceryl Topical Dermatophytes In solution (Lacquer); water-insoluble; Penetrate=↑Conc; Slow Cl MoA Two-Steps: Morpholine derivative that inhibits fungal sterol ∆14 reductase and sterol ∆7-8 isomerase (Zymosterol [Inh]Intermed.[Inh]Ergosterol) END OF MODULE 6