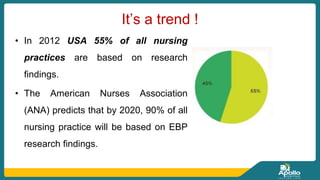
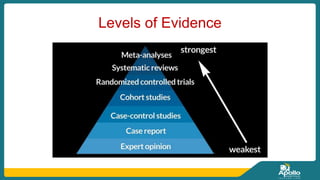
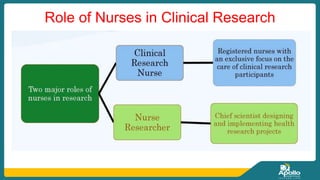
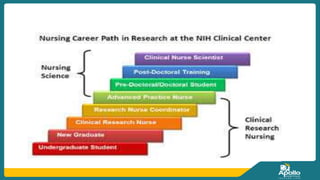
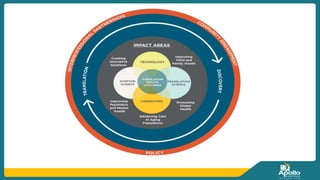
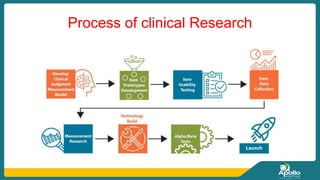
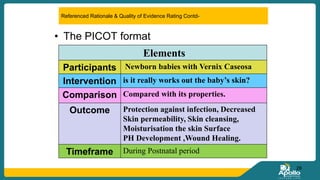
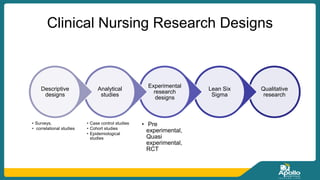
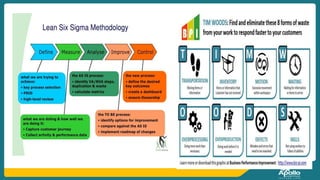
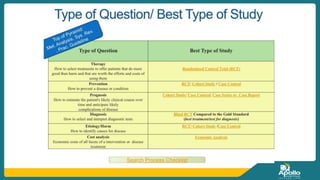
This document discusses clinical nursing research in India. It begins by stating that in 2012, 55% of nursing practices in the USA were based on research findings, and the ANA predicts this will rise to 90% by 2020. In India, key organizations that support nursing research include the Nursing Research Society of India and the Clinical Nursing Research Society. The document defines clinical nursing research and explains why it is important for evidence-based practice and improving patient outcomes. It also discusses the roles of nurses in clinical research, common research designs, and challenges in conducting this type of research.