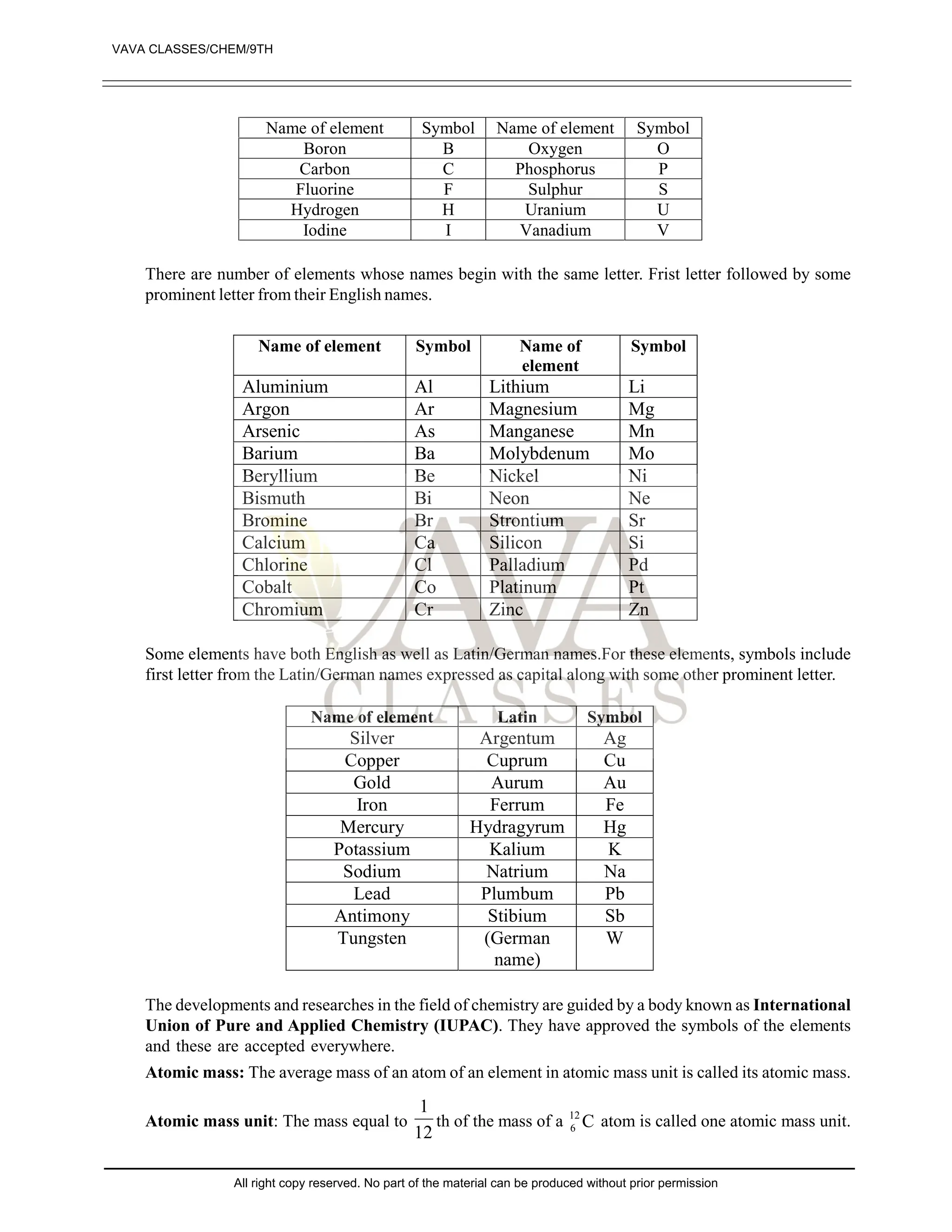
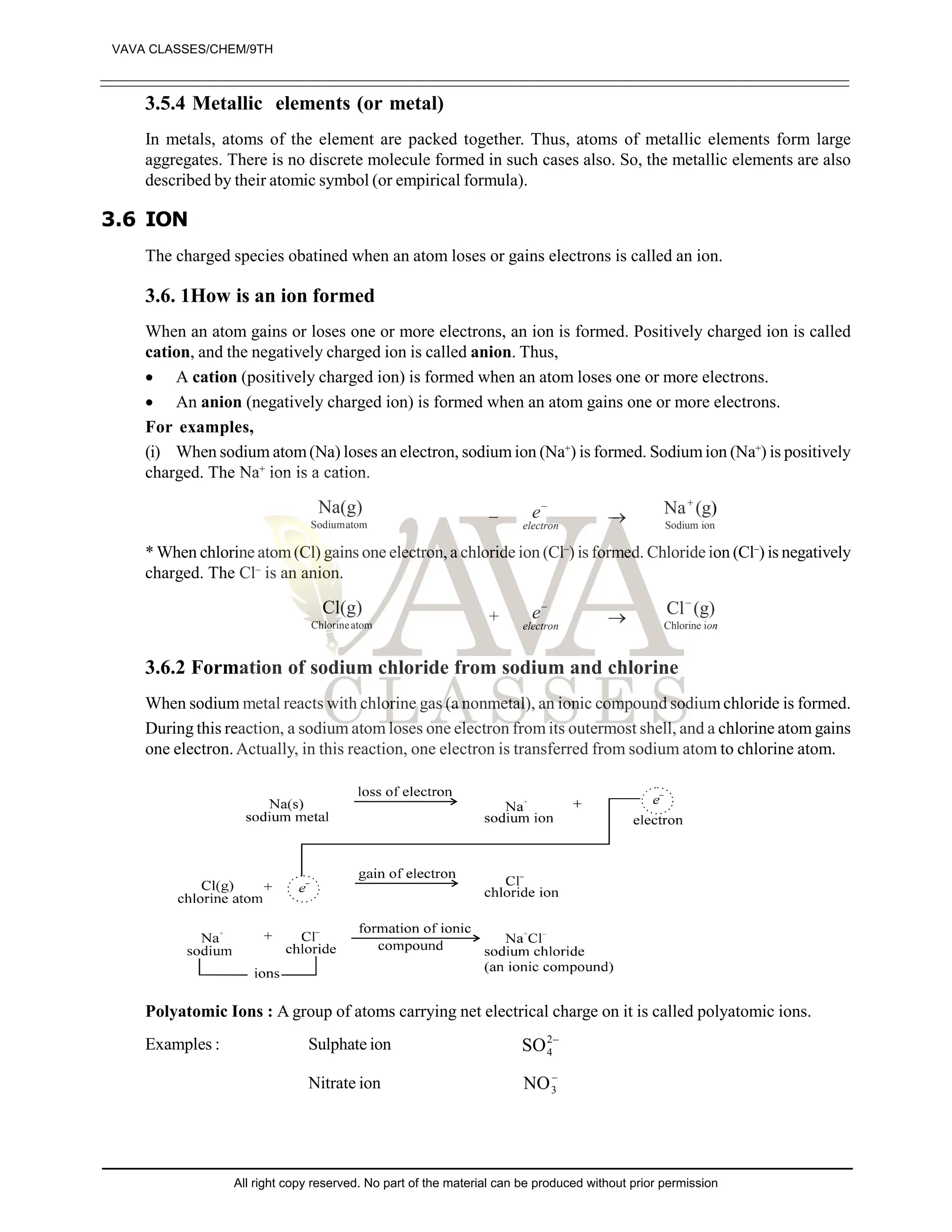
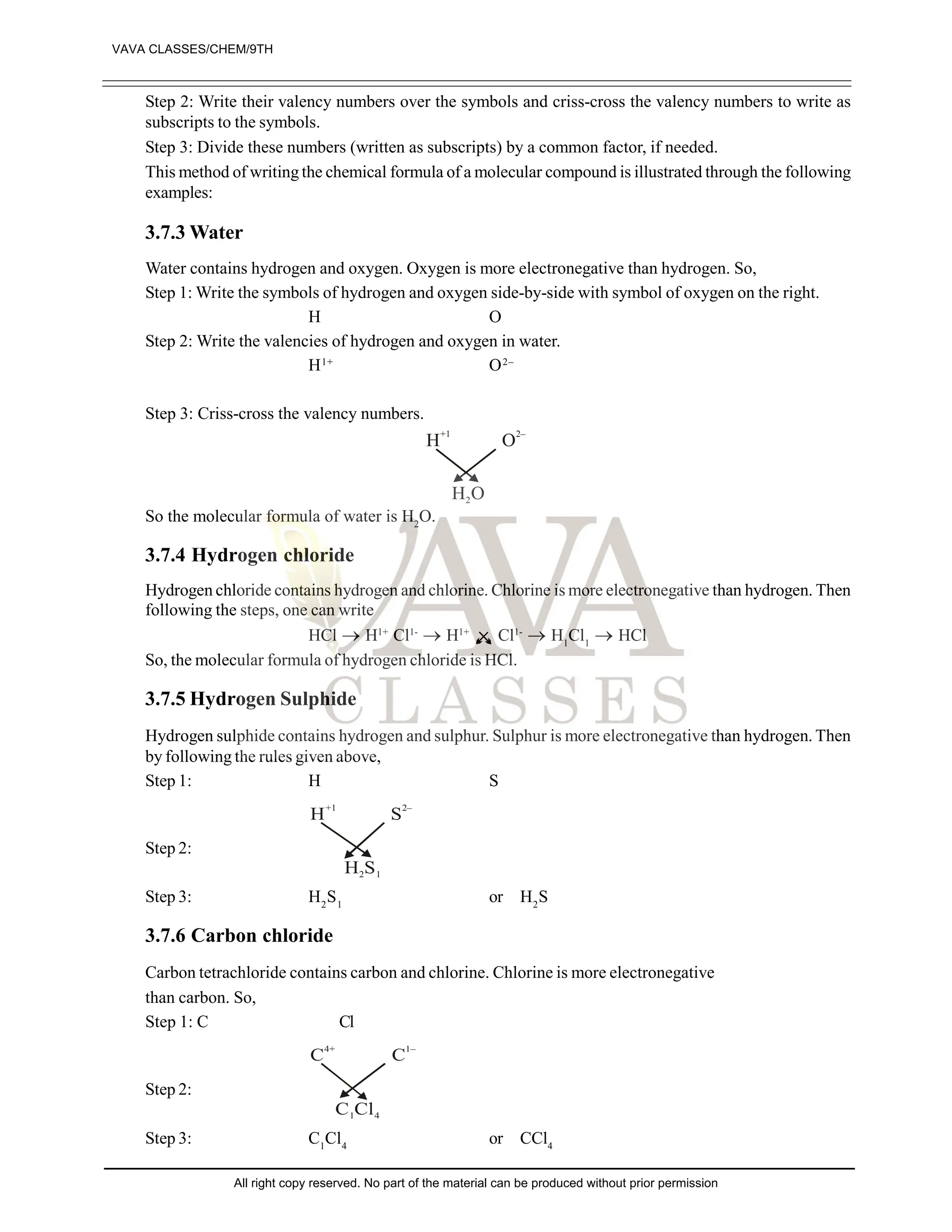
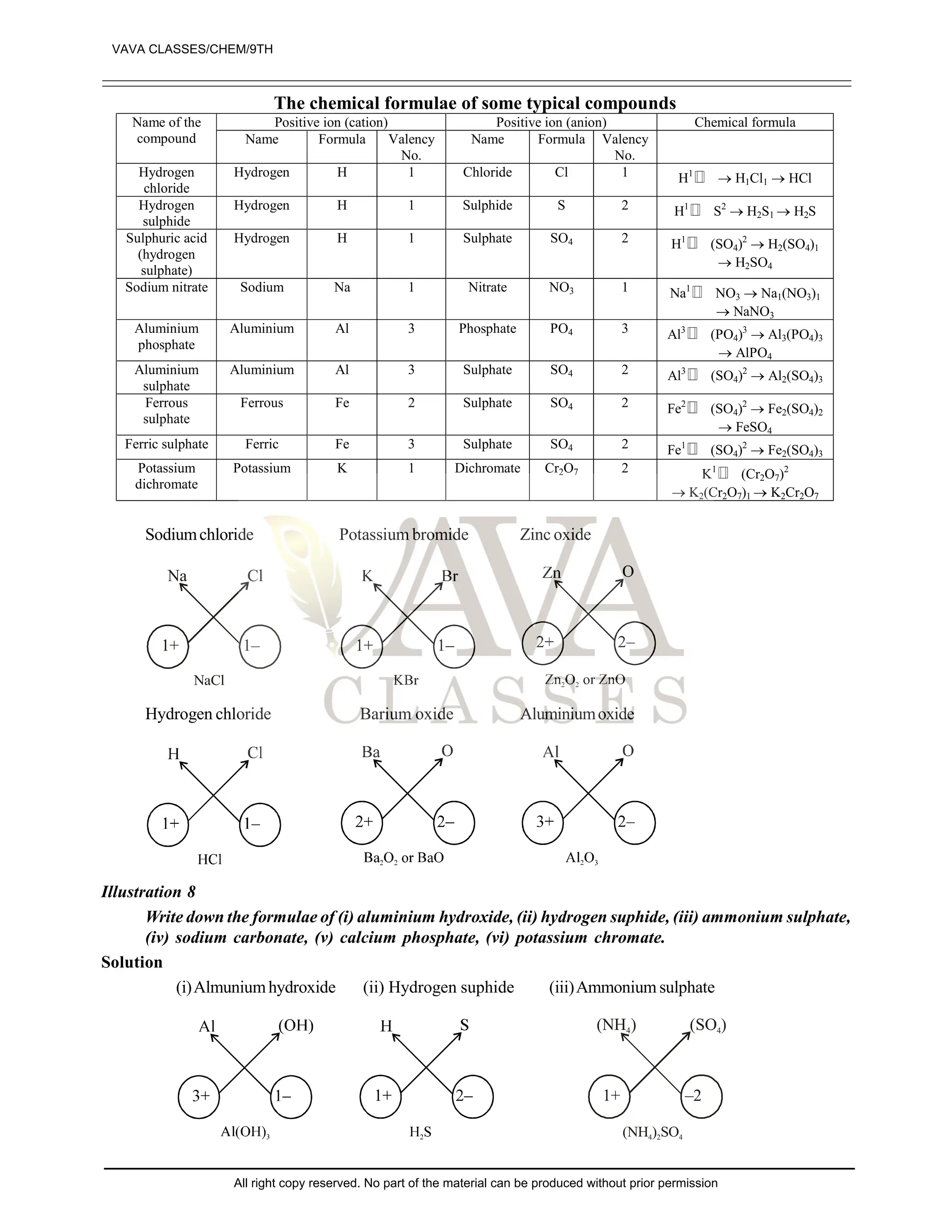
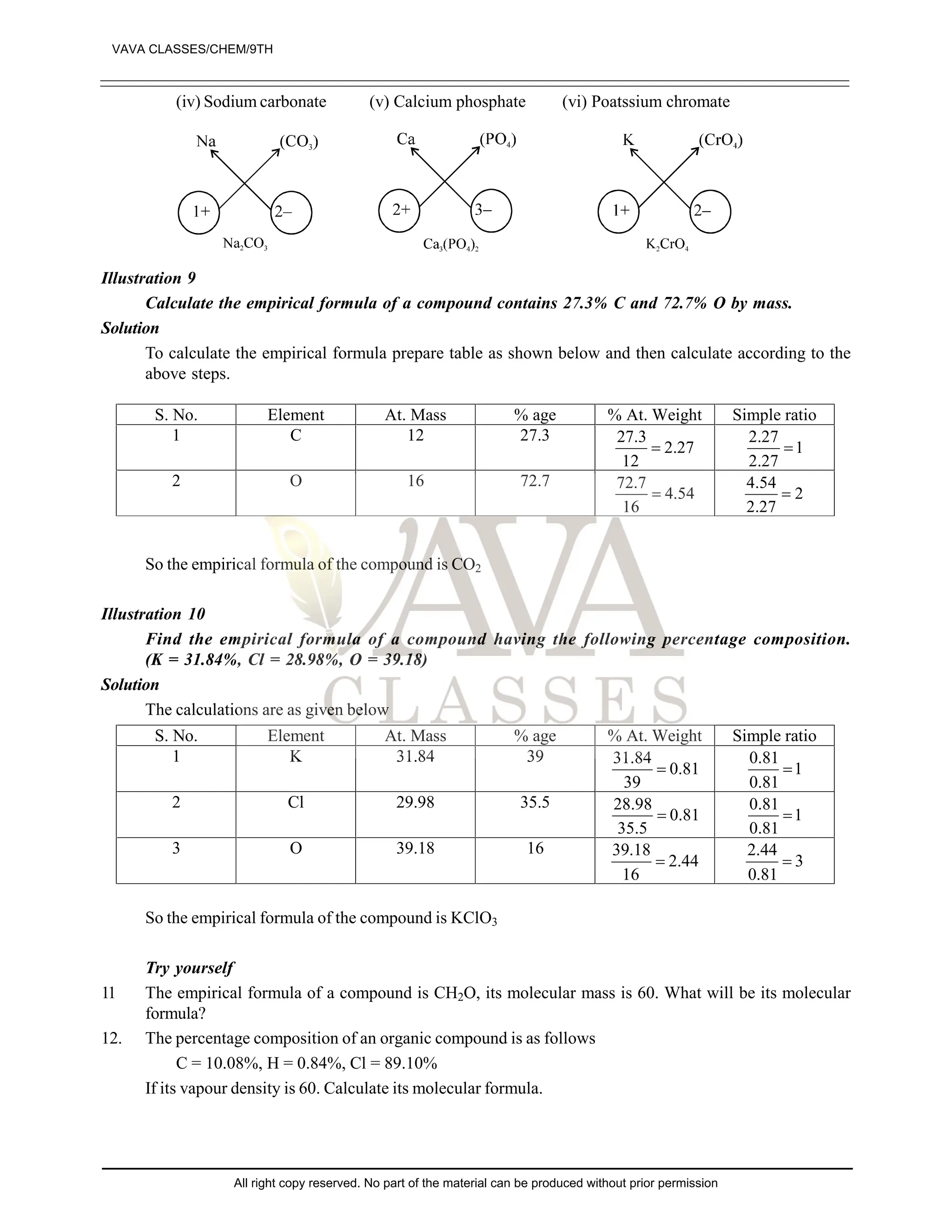
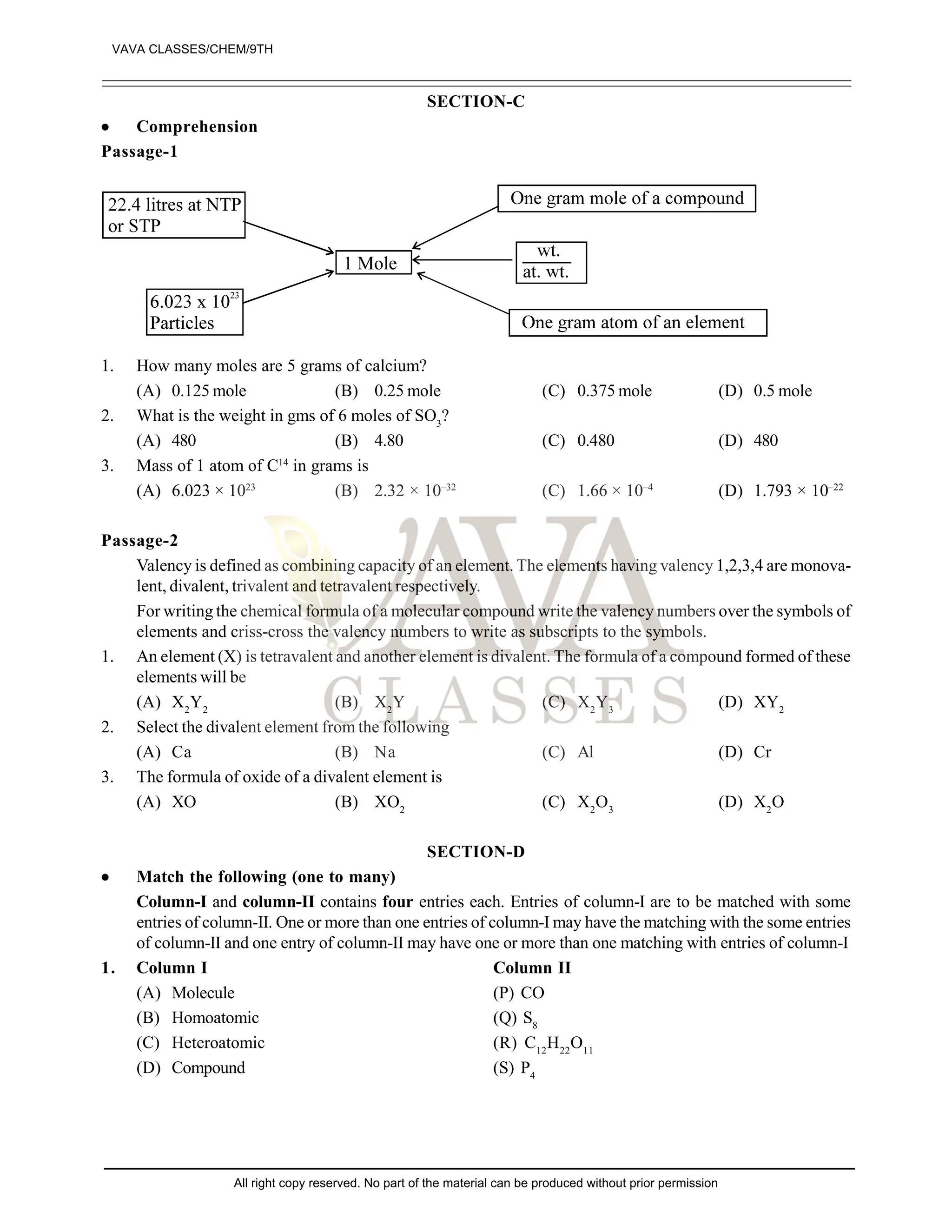
The document provides a comprehensive overview of atomic theory, including the historical perspective by Maharishi Kanad and key laws related to chemical combinations like the law of conservation of mass and the law of constant proportions. It also discusses Dalton's atomic theory, the structure of atoms and molecules, the concept of valency, and definitions related to chemical compounds. Various examples and illustrations reinforce the theoretical concepts presented.