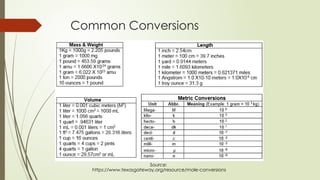
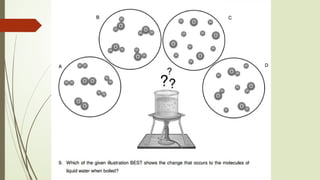
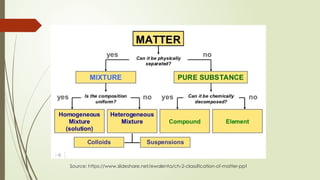
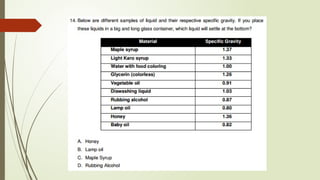
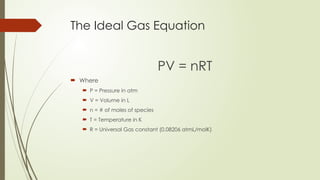
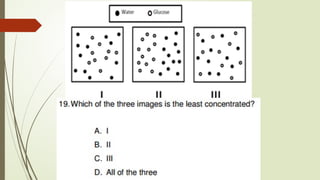
The document provides an overview of fundamental concepts in chemistry, including the study of matter, laws of conservation, and properties of matter. It discusses intermolecular forces, the ideal gas equation, and trends in the periodic table, as well as solutions and factors affecting solubility. Key laws such as Boyle’s, Charles’, and Avogadro’s are also outlined.