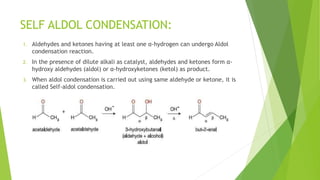
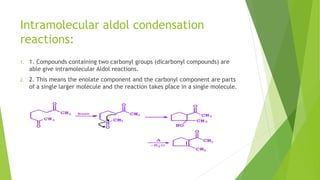
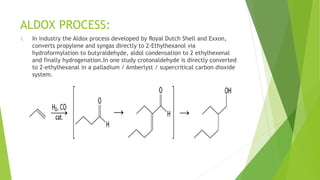
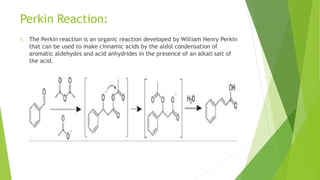
This document discusses various aldol condensation reactions and their mechanisms. It introduces crossed aldol condensation which produces up to four products from two different carbonyl compounds. Self-aldol condensation uses a single aldehyde or ketone. Intramolecular aldol condensation occurs when a molecule contains two carbonyl groups. Several industrial reactions are also summarized, including the Aldox process, Perkin reaction, and Meerwein-Ponndorf-Verley reaction. In conclusion, these reactions are reversible and complete conversion can be achieved through excess alcohol or acetone removal.