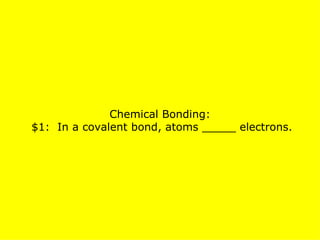
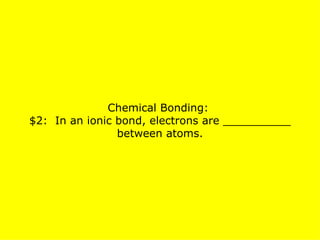
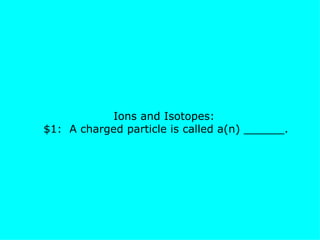
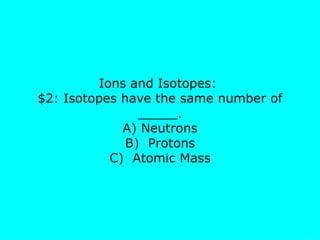
The document presents a chemistry jeopardy game format containing questions and answers on various topics such as the periodic table, atoms, elements, compounds, mixtures, chemical bonding, ions, and isotopes. It includes categories with questions ranked by difficulty and corresponding monetary values. The content is structured to facilitate learning and review of key chemistry concepts for test preparation.