1) The document discusses various topics related to chemical kinetics including reaction order, molecularity, and rate laws for zero-order, first-order, and second-order reactions.
2) It provides examples and characteristics of zero-order reactions where the rate is independent of concentration and the rate law is equal to the rate constant. It also discusses calculating half-life and shelf life for zero-order reactions.
3) First-order reactions are described where the rate is directly proportional to the concentration of one reactant and the integrated rate law results in an exponential decay of concentration over time. Half-life calculations for first-order reactions are also covered.
4) Second-order reactions have the rate directly proportional


![INTRODUCTION
Where,
Positive (+) sign- Increase in concentration over a period of time.
Negative (-) sign – Decrease in concentration with respect to time.
In general, a chemical reaction for kinetic study is written as
cC + dD Products
Rate =−
1
𝑐
𝑑[𝐶]
𝑑𝑡
Rate =−
1
𝑑
𝑑[𝐷]
𝑑𝑡
Gourav Singh
3](https://image.slidesharecdn.com/chemicalkinetics-210601100808/85/Chemical-kinetics-3-320.jpg)
![INTRODUCTION
Rate = 𝑘[𝐶]𝑐
[𝐷]𝑑
Where
K- rate constant or specific constant
[C]and [D] – molar concentration of C and D respectively.
Rate constant express the relationship between the rate of chemical reaction
and the concentration of the reacting substances.
Gourav Singh
4](https://image.slidesharecdn.com/chemicalkinetics-210601100808/85/Chemical-kinetics-4-320.jpg)

![ORDER OF REACTION
1) The sum of exponents or power of concentration term in the rate equation is
known as order of reaction.
2) Let us consider a general reaction.
𝑅𝑎𝑡𝑒 = 𝐾[𝐶]𝑐
[𝐷]𝑑
Thus the above order reaction is (c+d)
a) If the value of (c+d) is 0, then it is called zero order reactions.
b) If the value of (c+d) is 1, then it is called first order reactions.
c) If the value of (c+d) is 2, then it is called second order reactions.
Gourav Singh
7](https://image.slidesharecdn.com/chemicalkinetics-210601100808/85/Chemical-kinetics-7-320.jpg)

![ZERO ORDER REACTION
Derivatives
The rate of zero order reaction is expressed as
−𝑑𝐴
𝑑𝑡
On integrate equation
= − 𝐴0
𝐴𝑡
𝑑𝐴 = 𝐾 0
𝑡
𝑑𝑡
= − 𝐴0
𝐴𝑡
𝐴 = 𝐾 0
𝑡
[𝑡]
Gourav Singh
10](https://image.slidesharecdn.com/chemicalkinetics-210601100808/85/Chemical-kinetics-10-320.jpg)
![ZERO ORDER REACTION
= 𝐴0 − 𝐴𝑡 = 𝑘(𝑡 − 0)
= 𝐴0 − 𝐴𝑡 = 𝑘𝑡
Or 𝑘 =
𝐴0−𝐴𝑡
𝑡
This is integrated rate of equation
t= 0 time (t)
Slope = -k
Concentration [A]
Plot of concentration vs time
Gourav Singh
11](https://image.slidesharecdn.com/chemicalkinetics-210601100808/85/Chemical-kinetics-11-320.jpg)
![ZERO ORDER REACTION
Characteristics
1) Half life (𝒕𝟏/𝟐): It is the time required to reduce initial concentration of the
reactant to become half of its value during the progress of the reaction.
Initial concentration = 𝐴0
Final concentration = 𝐴0/2
By putting this value in equation [𝐴0 − 𝐴𝑡 = 𝑘𝑡] , we get:
𝑘𝑡1/2 = 𝐴0 −
𝐴0
2
𝑘𝑡1/2 =
𝐴0
2
Gourav Singh
12](https://image.slidesharecdn.com/chemicalkinetics-210601100808/85/Chemical-kinetics-12-320.jpg)
![ZERO ORDER REACTION
𝑡1/2 =
𝐴0
2𝑘
Half life is directly proportional to the initial concentration of reactant.
2) Shelf life: It is a time required for reactant concentration to decrease to 90% of
the initial concentration.
𝐴𝑡 = 0.9 𝐴0
By putting this value in equation [𝐴0 − 𝐴𝑡 = 𝑘𝑡] , we get:
𝑡0.9 =
𝐴0 −0.9 𝐴0
𝑘
Gourav Singh
13](https://image.slidesharecdn.com/chemicalkinetics-210601100808/85/Chemical-kinetics-13-320.jpg)
![ZERO ORDER REACTION
𝑡0.9 =
0.1 𝐴0
𝑘
This unit of k for zero order reaction is moles/litre/second
Example
1. Photochemical reaction between hydrogen and chlorine.
𝐻2 𝑔 + 𝐶𝑙2 𝑔
ℎ𝑣
2𝐻𝐶𝐿 (𝑔)
2. Decomposition of 𝑵𝟐𝑶 on a hot platinum surface.
𝑁2𝑂 → 𝑁2 +
1
2
𝑂2
𝑅𝑎𝑡𝑒 ∝ [𝑁2𝑂]0
= 𝑘[𝑁2𝑂]0
= 𝑘
Gourav Singh
14](https://image.slidesharecdn.com/chemicalkinetics-210601100808/85/Chemical-kinetics-14-320.jpg)
![ZERO ORDER REACTION
𝑑[𝑁2𝑂]
𝑑𝑡
= 𝑘
3. Decomposition of 𝑵𝑯𝟑 in the presence of molybdenum or tungsten.
2𝑁𝐻3
[𝑀𝑜]
𝑁2 + 3𝐻2
Problems
I. What is the value of rate constant if [𝐴0] = 2.30M and half life is 7.30 min.
Ans:- 𝑡1/2 = 𝐴0/2𝑘
k=2.3 M/2 (7.3min)
k = 0.157 mol/1.min
Gourav Singh
15](https://image.slidesharecdn.com/chemicalkinetics-210601100808/85/Chemical-kinetics-15-320.jpg)

![PSEUDO ORDER REACTION
An order of a chemical reaction that appears to be less than the true order due to
experimental conditions; when a one reactant is in large excess.
There are two types of reaction
1) Pseudo first order reaction
2) Pseudo second order reaction
FIRST ORDER REACTION SECOND ORDER REACTION
Pseudo first order kinetics 2nd order rate law
= k [A] or [B]
Pseudo second order kinetic 3rd order rate
law = 𝑘[𝑎]2[𝐵]
Reduce to first Pseudo first order if either [a]
or [B] in large excess
Reduce to pseudo first order if [A] is in
excess, Pseudo second order if [B] is in
Gourav Singh
17](https://image.slidesharecdn.com/chemicalkinetics-210601100808/85/Chemical-kinetics-17-320.jpg)
![FIRST-ORDER REACTION
First order reaction is defined as a reaction in which the rate of reaction
depends on the concentration of one reactant.
Let us consider
𝐴 → 𝑝𝑟𝑜𝑑𝑢𝑐𝑡𝑠
Rate =𝑘[𝐴]1
Rate = -d[A]/dt
equate both above equation, we get
k[A] = -d[A]/dt
or k dt = -d[A]/[A]
Gourav Singh
18](https://image.slidesharecdn.com/chemicalkinetics-210601100808/85/Chemical-kinetics-18-320.jpg)
![FIRST-ORDER REACTION
Integrating the above equation
On integration
𝑘 0
𝑡
𝑑𝑡 = − 𝐴0
𝐴 𝑑𝐴
𝐴
𝑘0
𝑡
𝑡 = −𝐴0
𝐴𝑡
[𝑙𝑜𝑔𝑒 𝐴]
𝑘𝑡 = −[𝑙𝑜𝑔𝑒𝐴𝑡 − log 𝐴0]
𝑘𝑡 = − log
𝐴𝑡
𝐴0
As we know
log 𝑋𝑛 = 𝑛𝑙𝑜𝑔 𝑥
Gourav Singh
19](https://image.slidesharecdn.com/chemicalkinetics-210601100808/85/Chemical-kinetics-19-320.jpg)

![FIRST-ORDER REACTION
Or 𝐴𝑡 = 𝐴0𝑒−𝑘𝑡
For first order equation, when we plot concentration against time, a curve is
obtained.
The curve shows that concentration decrease exponentially with time.
The 𝑘 =
2.303
𝑡
log[
𝐴0
𝐴𝑡
] can be written as-
Gourav Singh
21](https://image.slidesharecdn.com/chemicalkinetics-210601100808/85/Chemical-kinetics-21-320.jpg)

![FIRST-ORDER REACTION
Half life:
To calculate half life
𝐴𝑡 =
𝐴0
2
As we know rate equation for first order reaction is
𝑘𝑡 = 2.303 log[
𝐴0
𝐴𝑡
]
By putting value of 𝐴𝑡 into above equation, we get
𝑘𝑡1/2 = 2.303 log[
𝐴0
𝐴0/2
]
Or 𝑘𝑡1/2 = 2.303 𝑙𝑜𝑔2
𝑡1/2 = 0.693/k
Gourav Singh
23](https://image.slidesharecdn.com/chemicalkinetics-210601100808/85/Chemical-kinetics-23-320.jpg)


![FIRST-ORDER REACTION
II. The initial concentration of drug was found to be 0.075 M. The concentration
after 12 hours was 0.055 M. Calculate the reaction rate constant if
decomposition of drug follows first order reaction.
Solution:- The first order reactions is
𝑘𝑡 = 2.303 log[
𝐴0
𝐴𝑡
]
𝑘 =
2.303
12
𝑙𝑜𝑔
0.075
0.055
k = 0.02527ℎ𝑟−1
Gourav Singh
26](https://image.slidesharecdn.com/chemicalkinetics-210601100808/85/Chemical-kinetics-26-320.jpg)
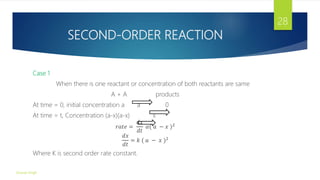
![SECOND-ORDER REACTION
The reaction is said to be second order when rate of reaction is directly
proportional to the concentration of two reactants.
In second order reaction two condition are possible
1) When
A Products
𝑑𝑥
𝑑𝑡 𝛼 [𝐴]2
2) When
A + B Products
𝑑𝑥
𝑑𝑡 𝛼 𝐴 𝐵
Gourav Singh
27](https://image.slidesharecdn.com/chemicalkinetics-210601100808/85/Chemical-kinetics-27-320.jpg)




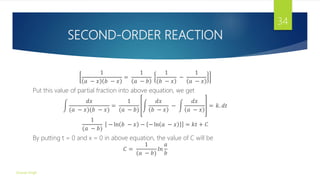



![DETRMINATION OF REACTION ORDER
Order of a reaction can be determine by any one of the following methods:
1) Substitution Method: In this method, the data obtained from a kinetic
experiment is substituted in the appropriate rate equation. The equation gives a
fairly constant value of k and indicates the order of a reaction. The rate and
half-life equation for different order reaction are given in table:-
Order Rate Law
0 Rate=k
1 Rate=k[A]
2 Rate=k[𝐴]2
Gourav Singh
38](https://image.slidesharecdn.com/chemicalkinetics-210601100808/85/Chemical-kinetics-38-320.jpg)
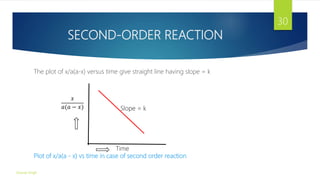
![DETRMINATION OF REACTION ORDER
2) Graphical Method: In this method,, the data obtained from a kinetics
experiment is plotted in the appropriate form for determining the order of a
reaction. For Example,
i. If a plot of concentration versus time (t) yield a straight line, the reaction is of
zero-order.
ii. If a plot of log (a - x)versus t yield a straight line, the reaction is of first –order.
iii. If a plot of
𝑥
[𝑎 𝑎 −𝑥 ]
versus t yields a straight line (provided the initial
concentration are equal), the reaction is of second-order.
Gourav Singh
39](https://image.slidesharecdn.com/chemicalkinetics-210601100808/85/Chemical-kinetics-39-320.jpg)
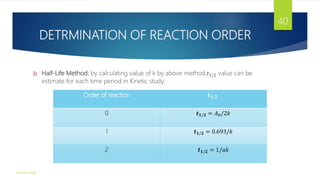





![SUMMARY OF THE KINETICS
Order Rate Law Concentration-
time
Equation
Half –Life M/t Unit of k
0 Rate=k [A] = [𝐴]0 - kt
𝑡1
2
=
[𝐴]0
2𝑘
k m/s, m/min,
m/hrs, etc
1 Rate=k[A] In[A] = In[𝐴]0 -
kt
𝑡1
2
=
𝐼𝑛2
𝑘
kM 𝑠−1, 𝑚𝑖𝑛−1, ℎ𝑟−1,
𝑒𝑡𝑐.
2 Rate=k[𝐴]2 1
[𝐴]
=
1
[𝐴]0
+ 𝑘𝑡 𝑡1
2
=
1
𝑘 𝐴 0
𝑘𝑀2
𝑚−1
𝑠−1
, 𝑚−1𝑚𝑖𝑛−1
𝑚−2
ℎ𝑟−1
, 𝑒𝑡𝑐.
Gourav Singh
46](https://image.slidesharecdn.com/chemicalkinetics-210601100808/85/Chemical-kinetics-46-320.jpg)
