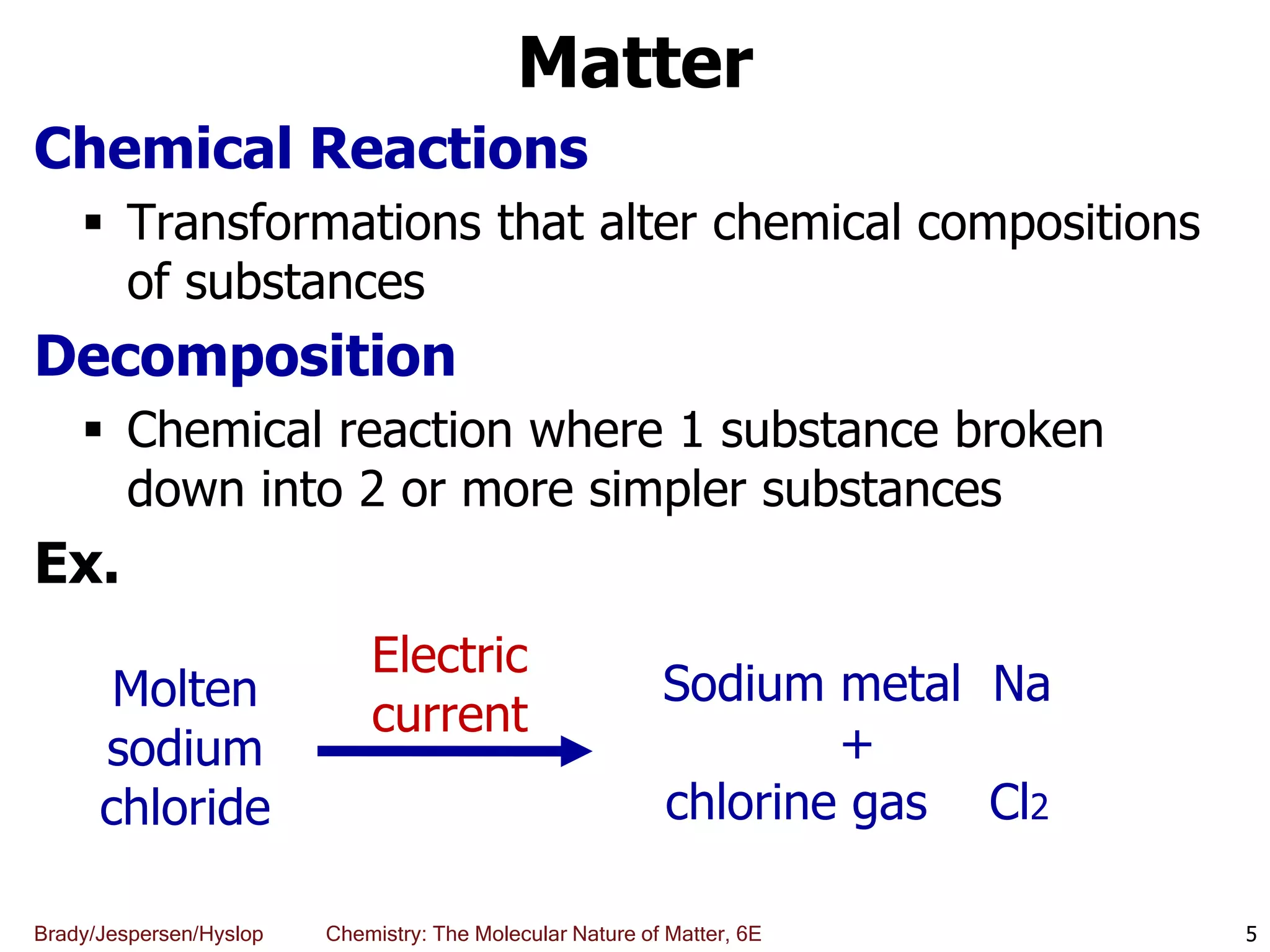
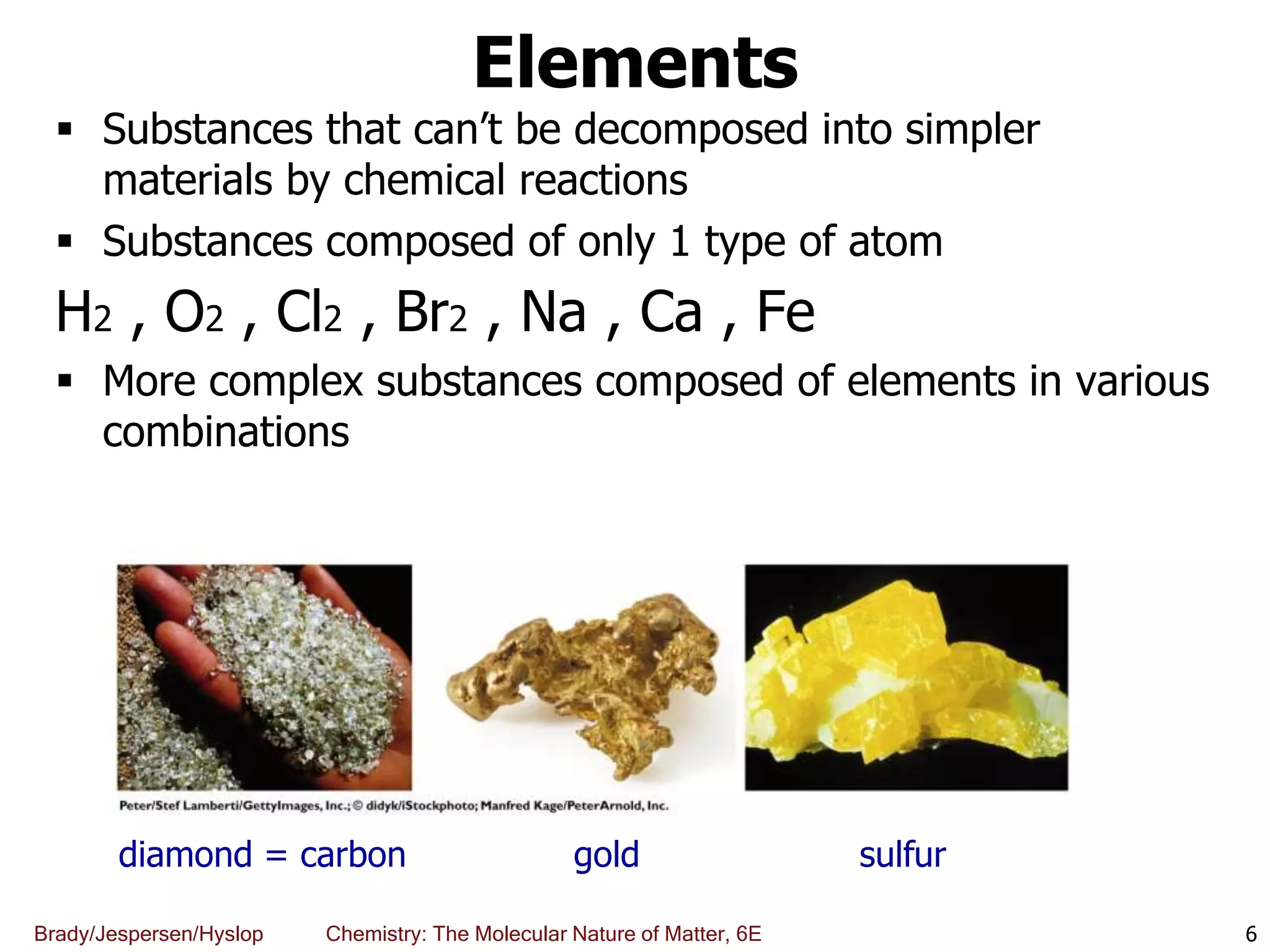
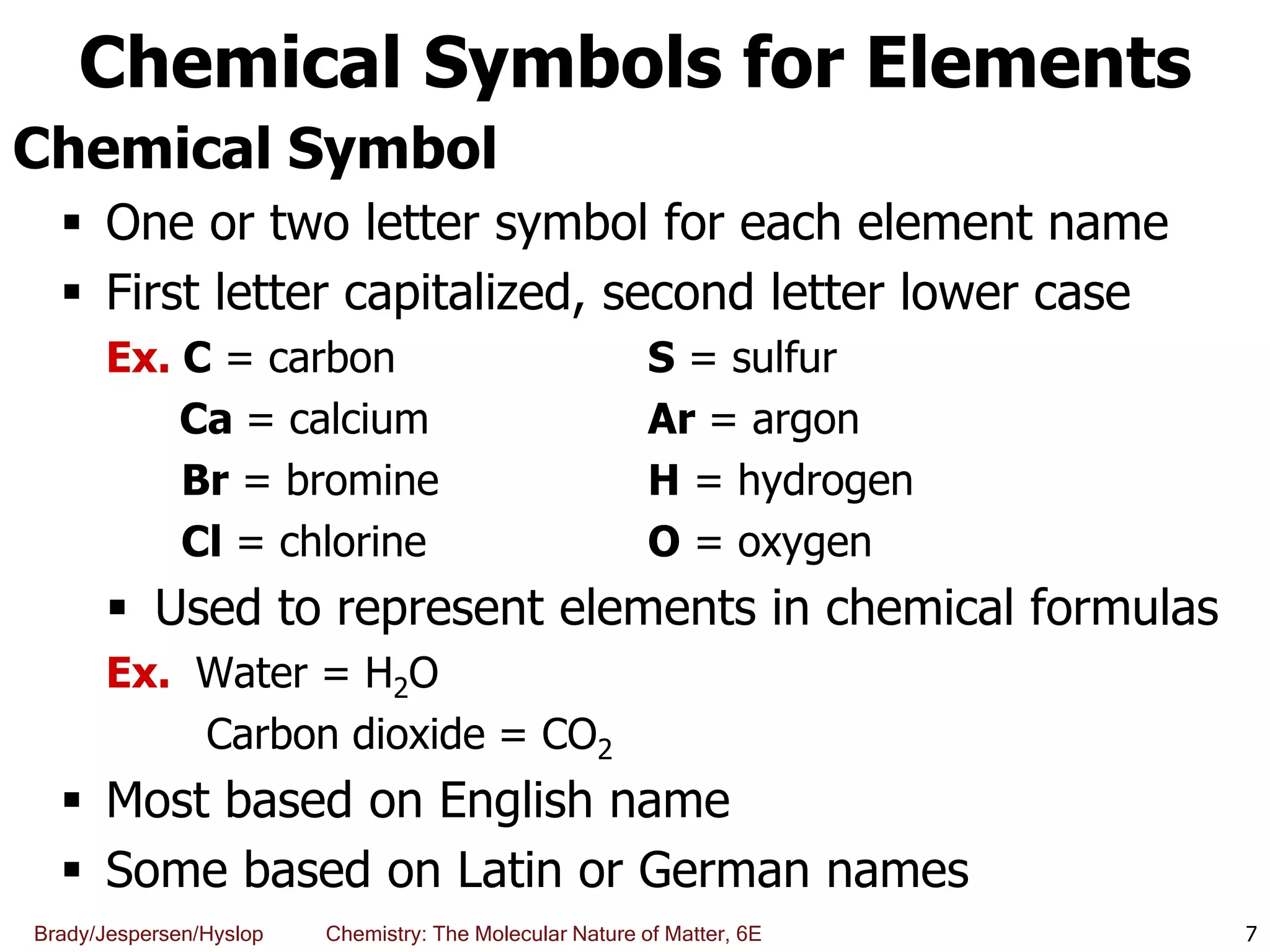
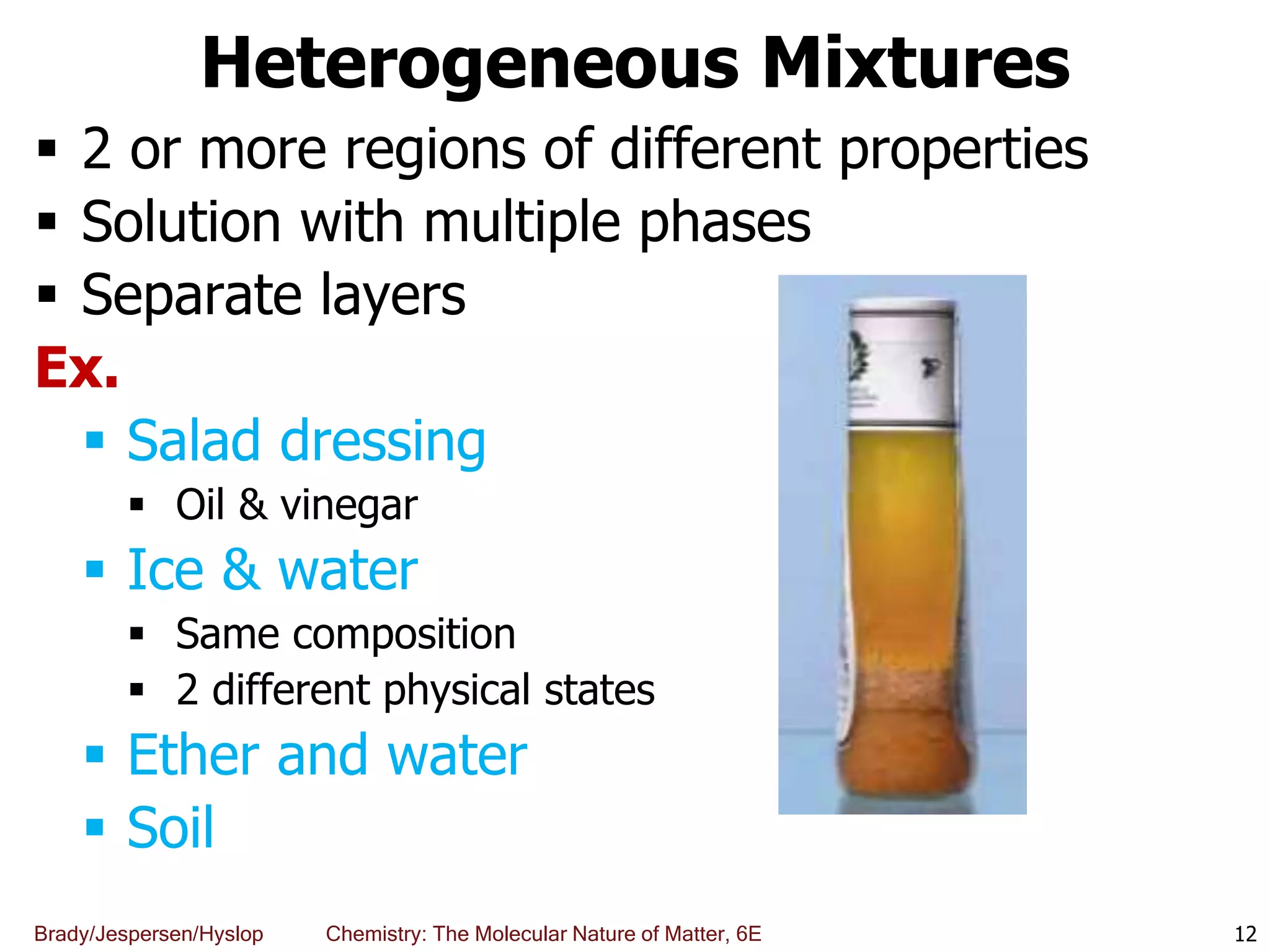
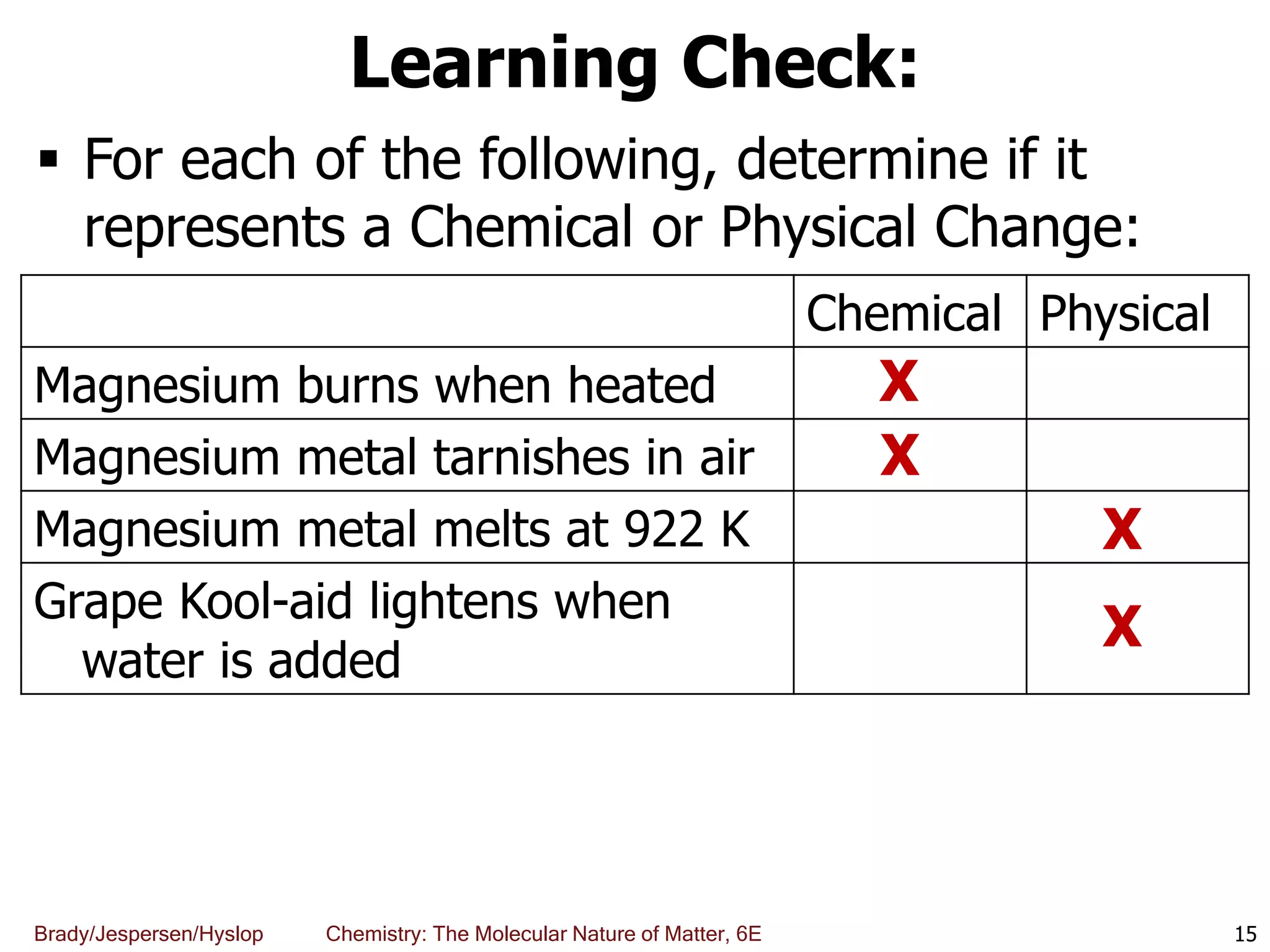
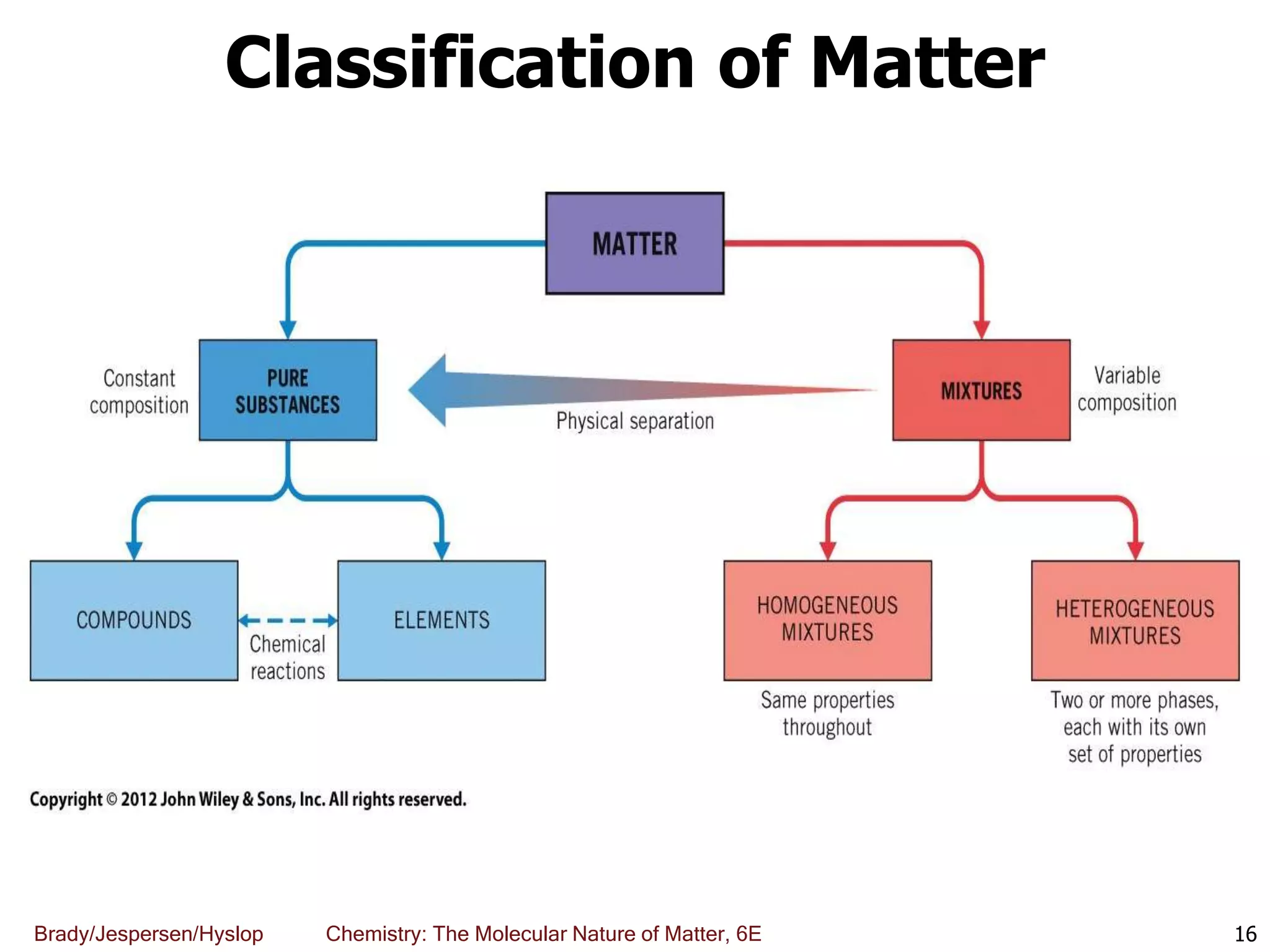
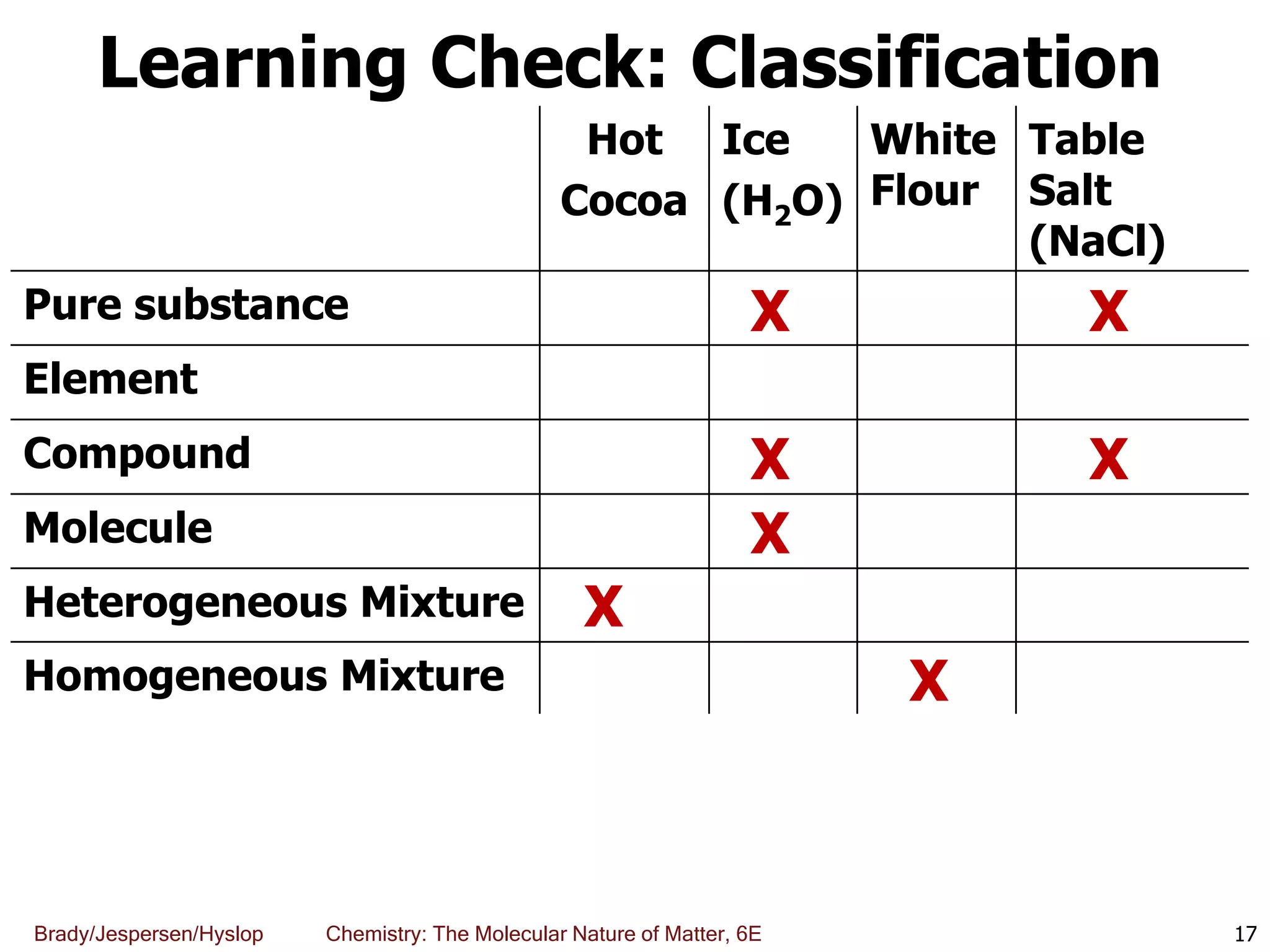
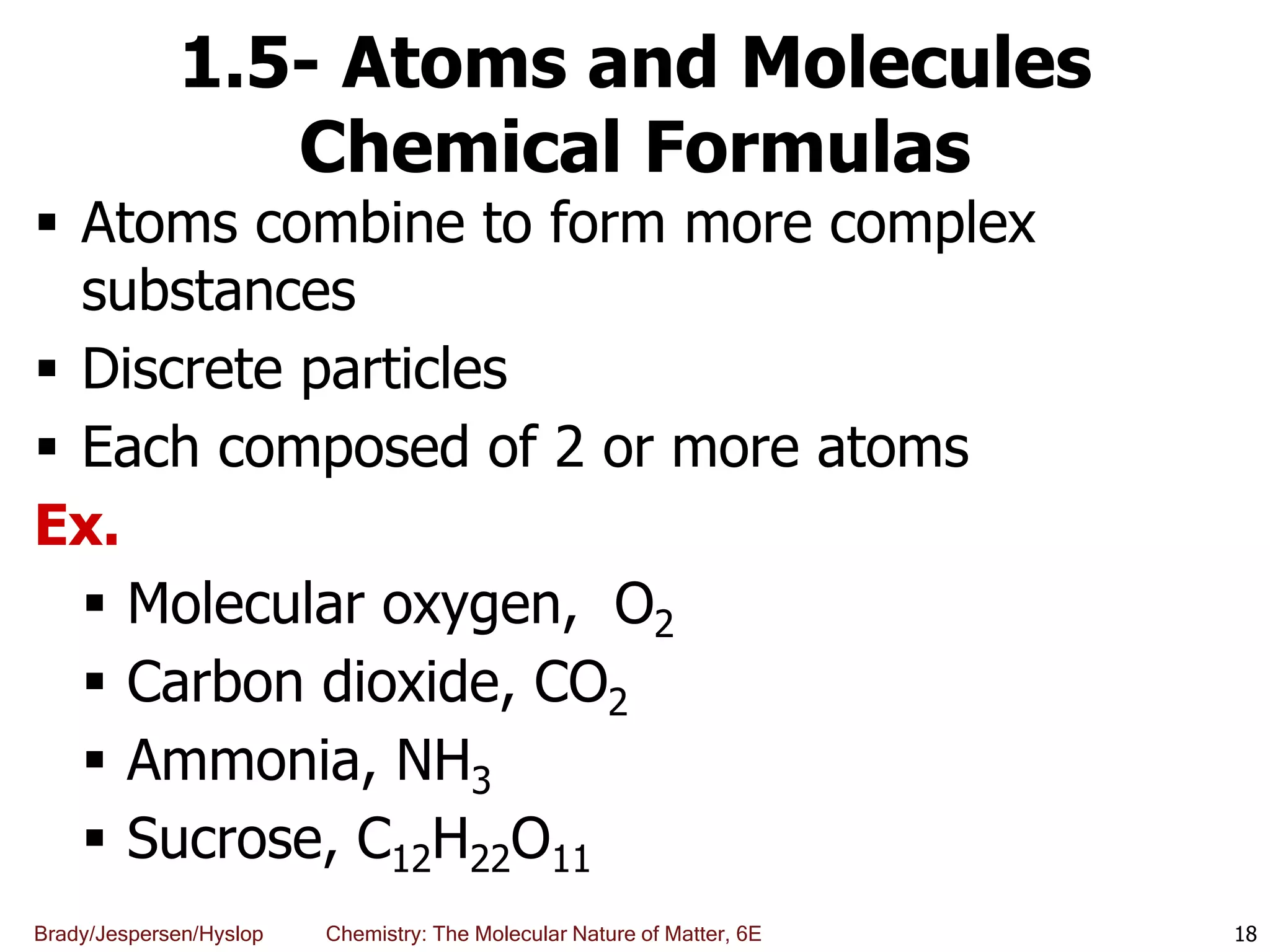
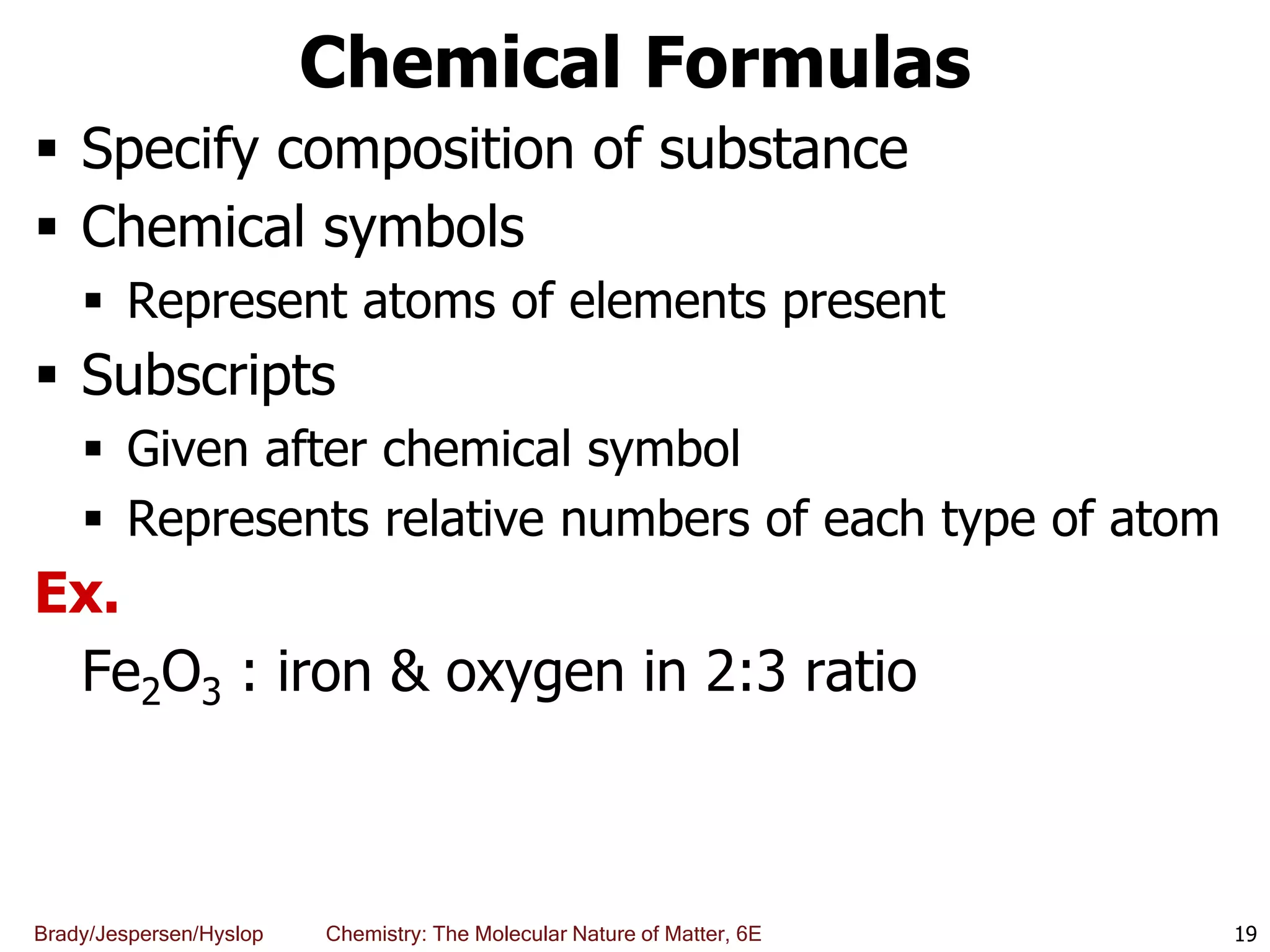
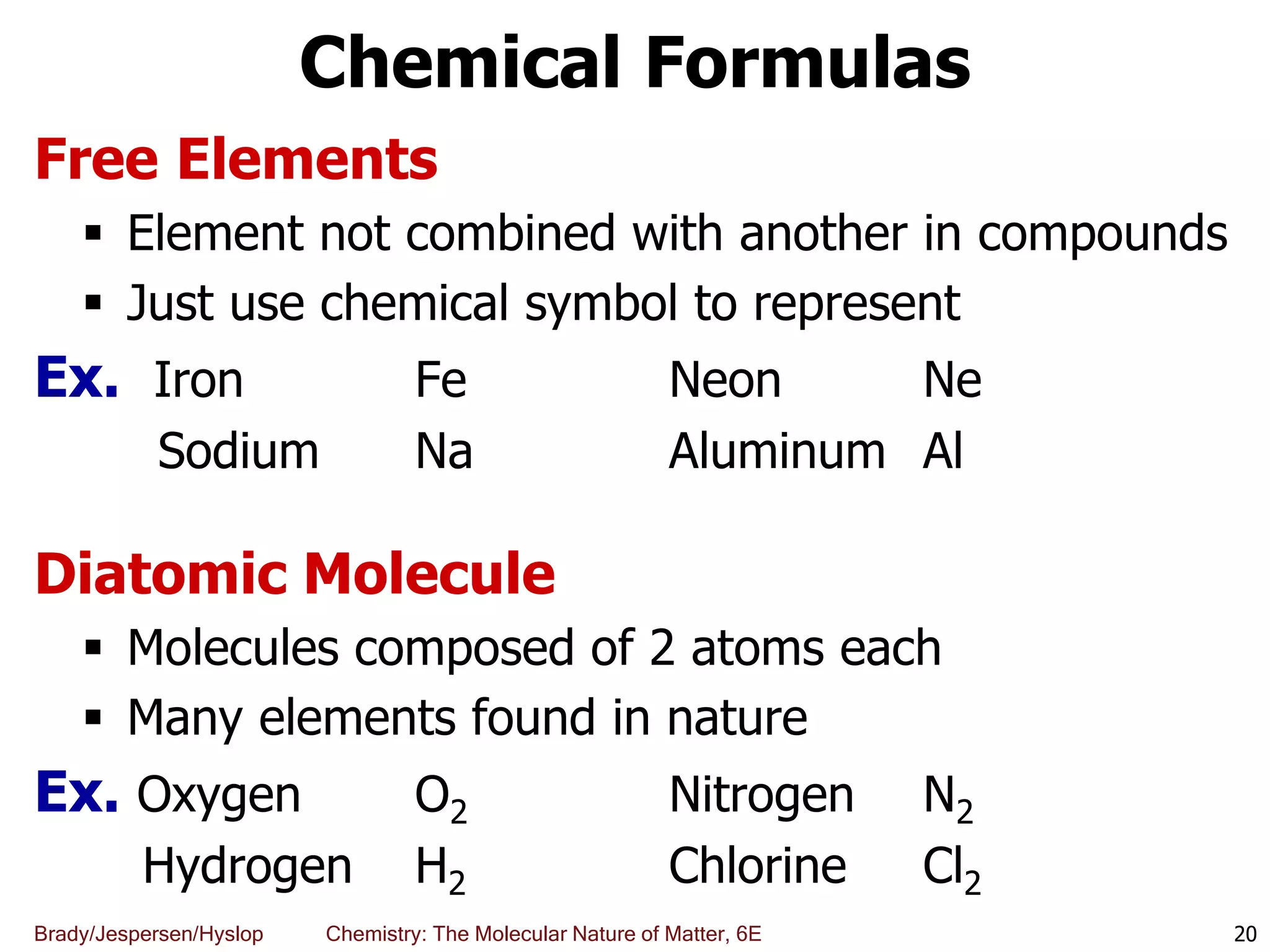
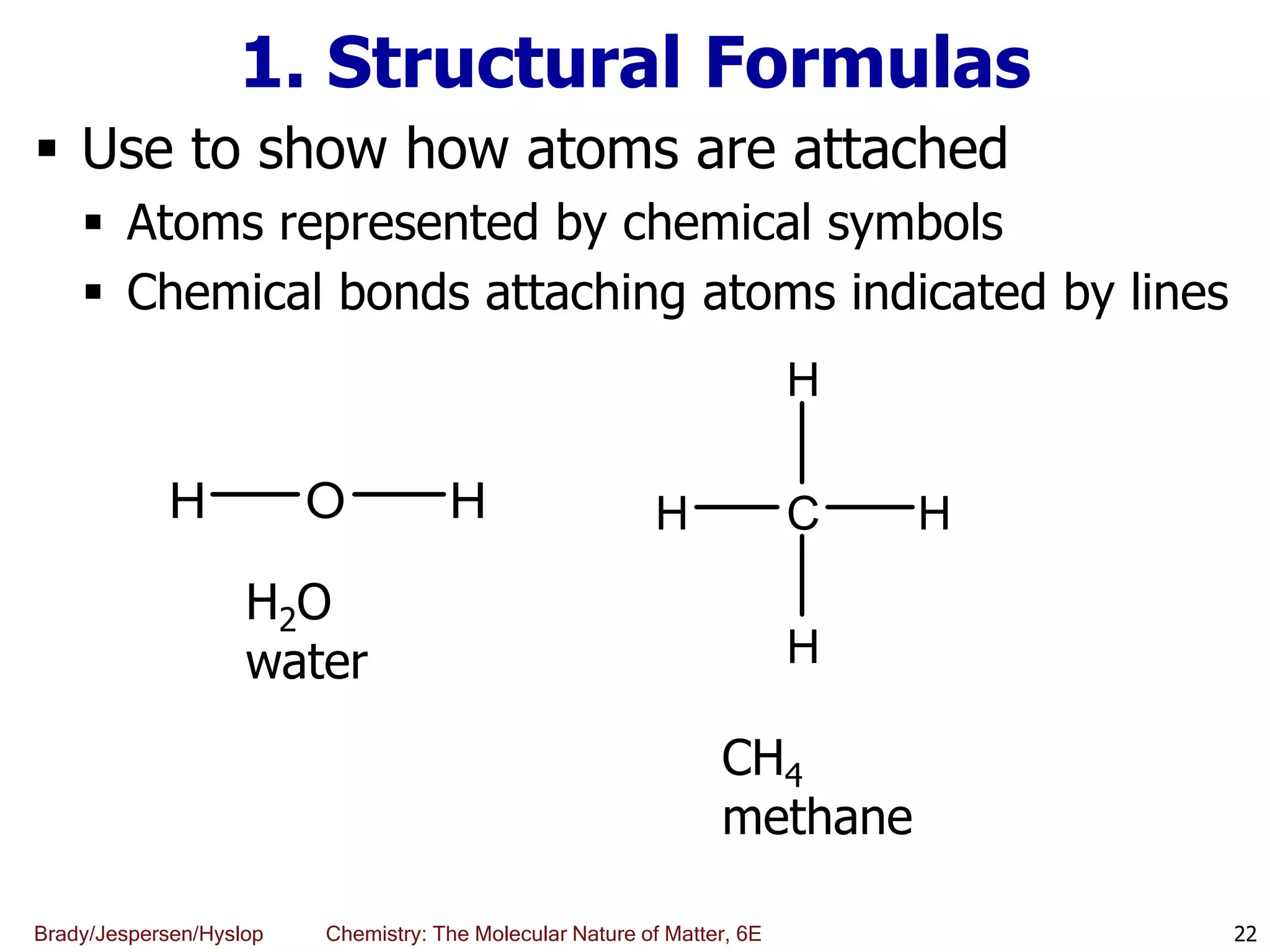
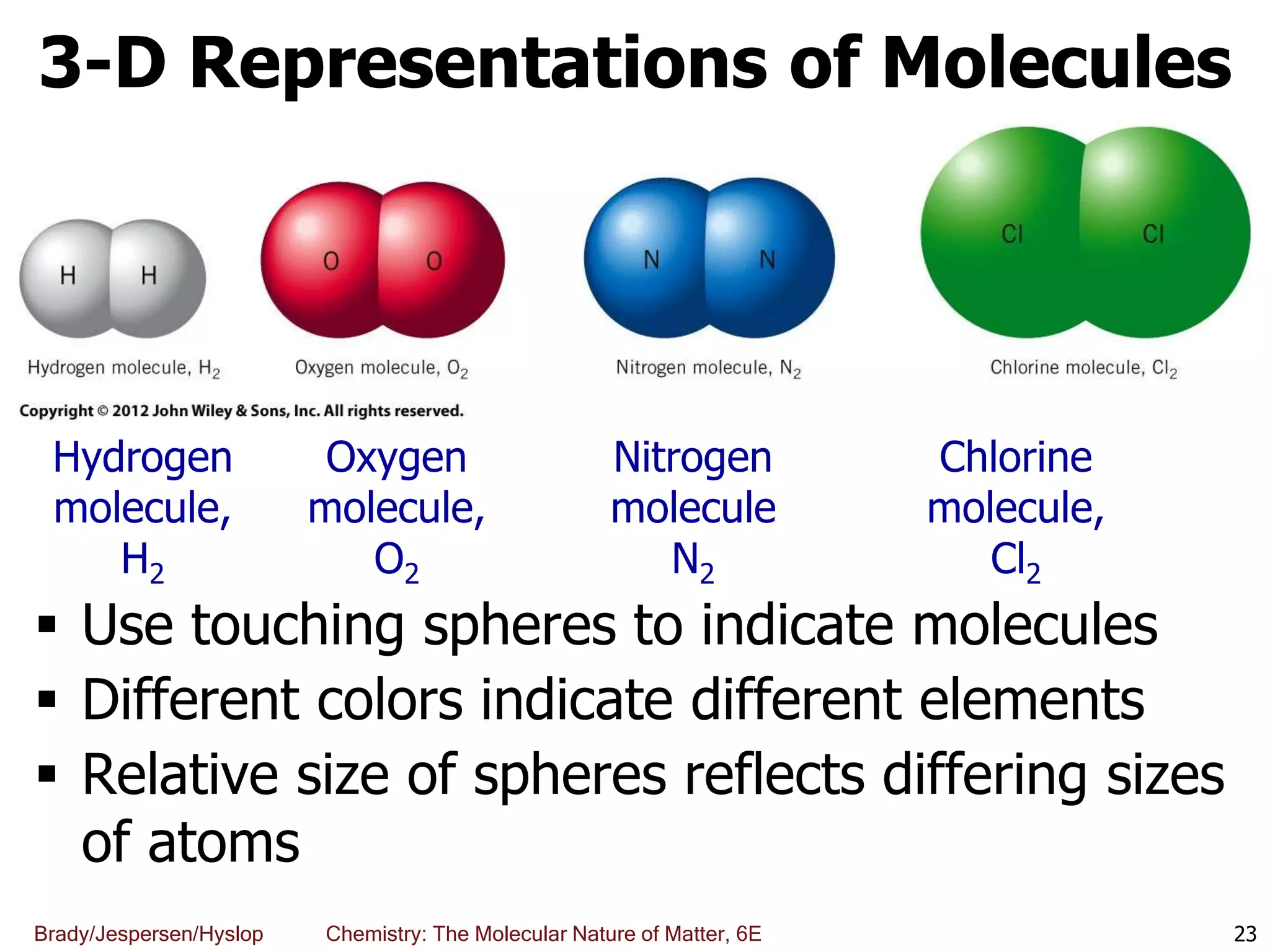
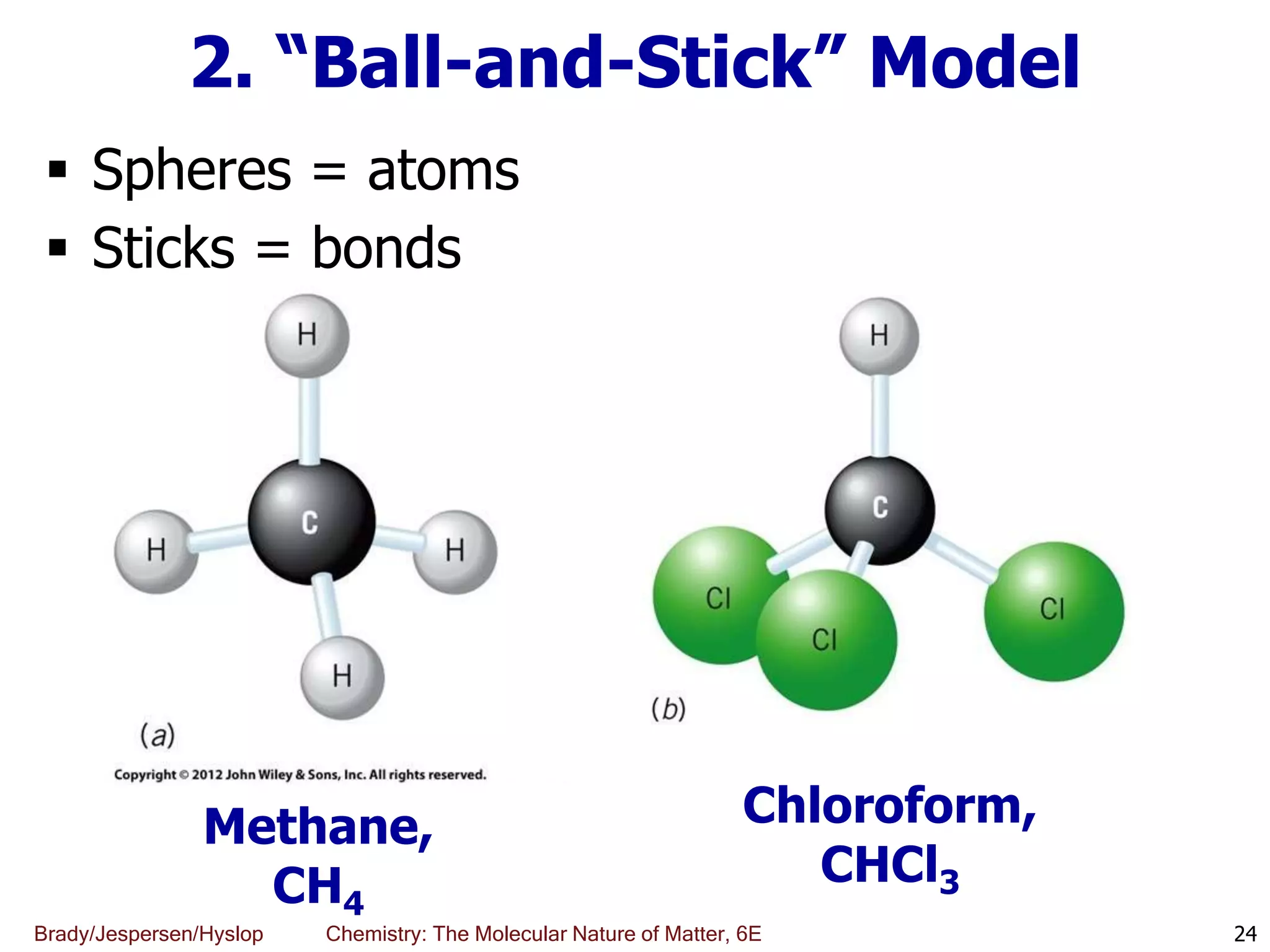
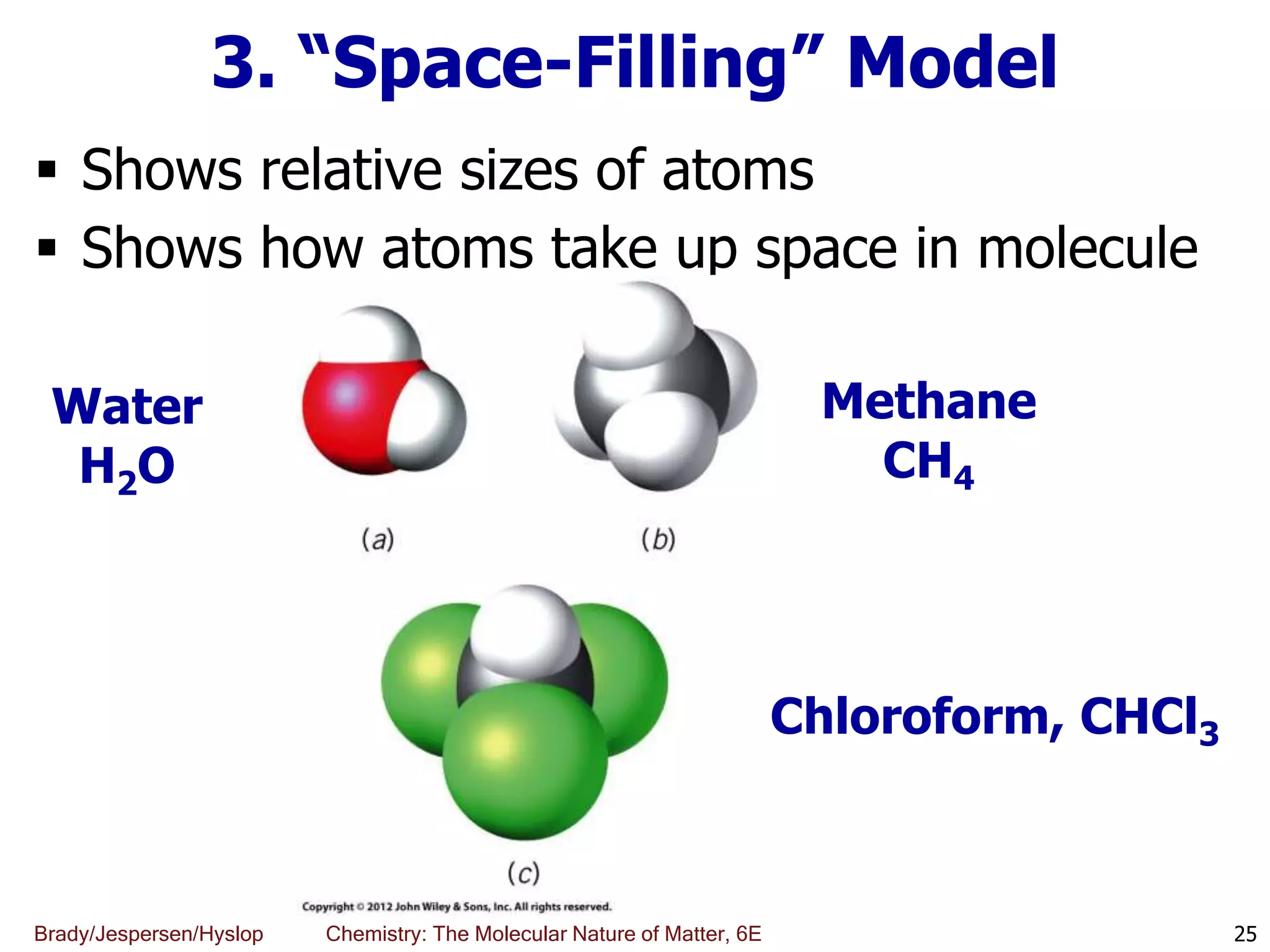
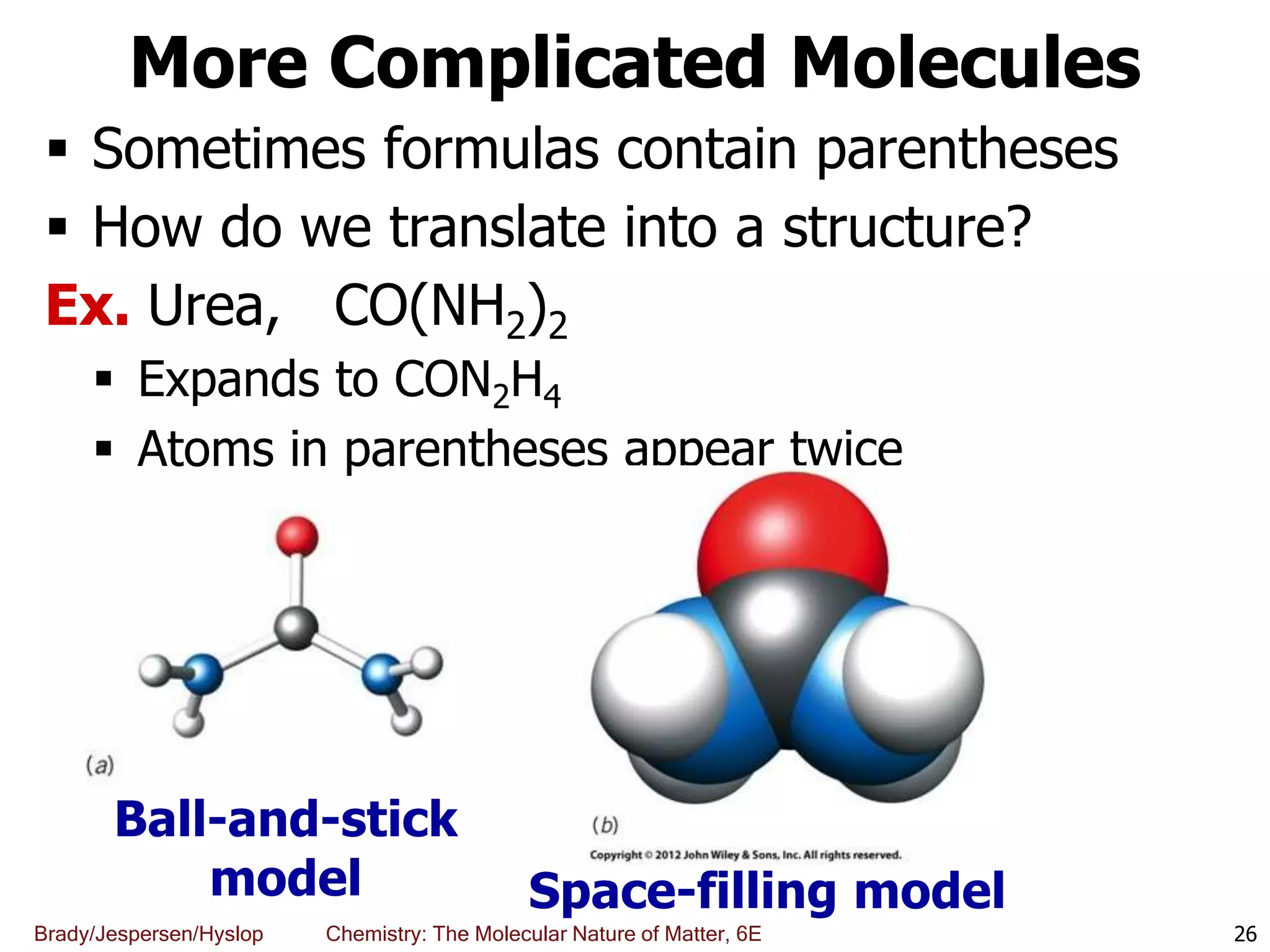
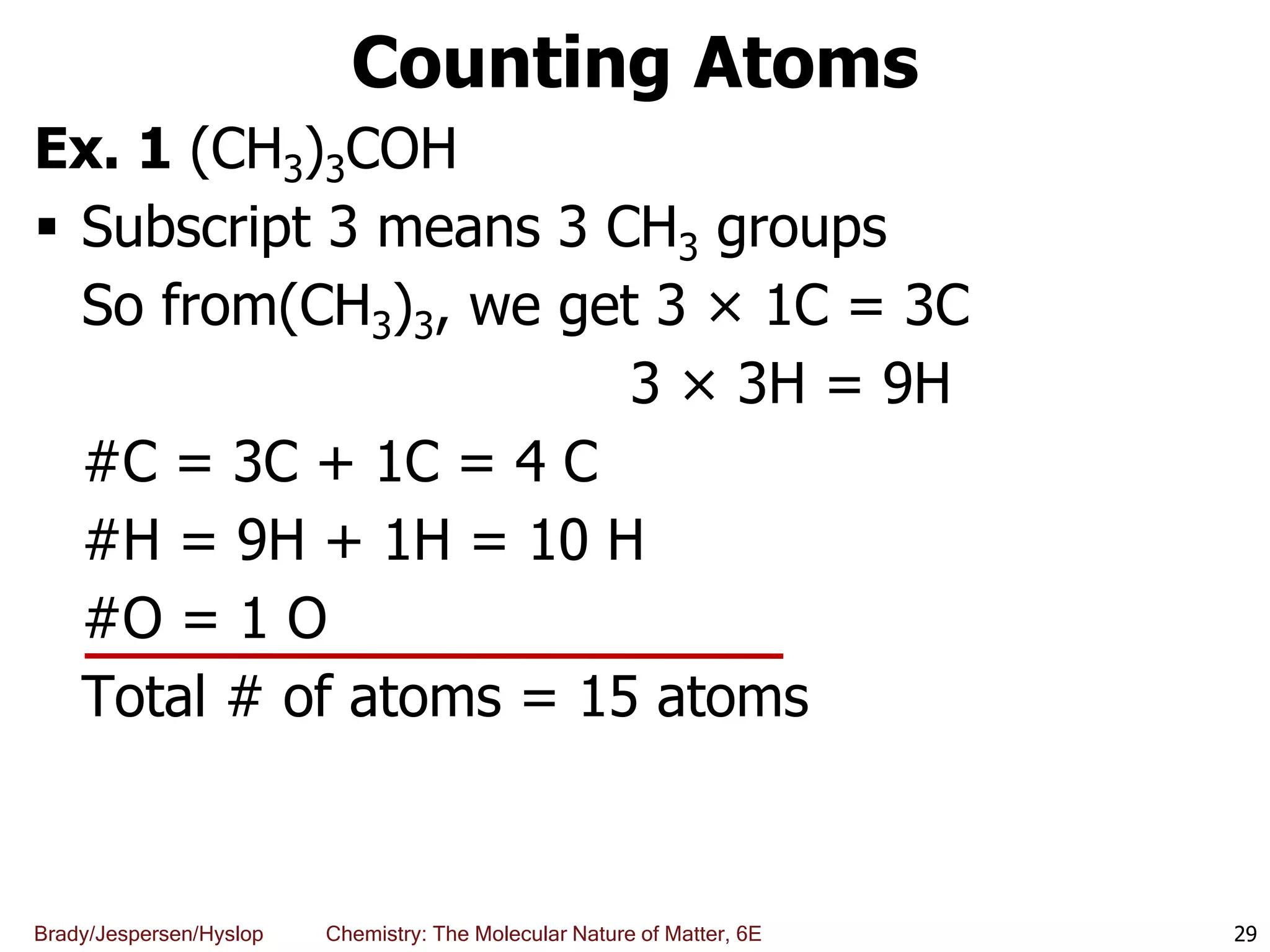
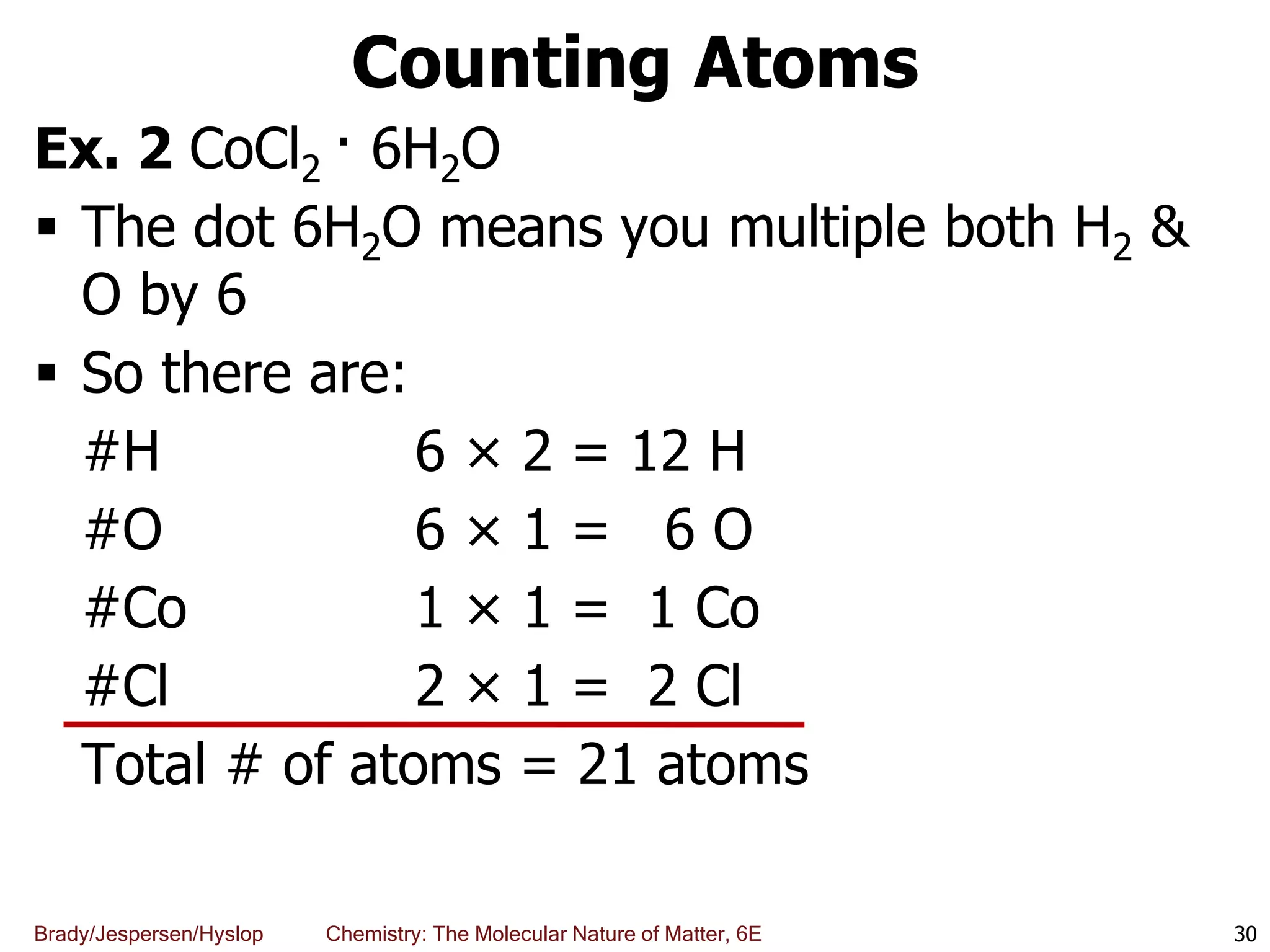
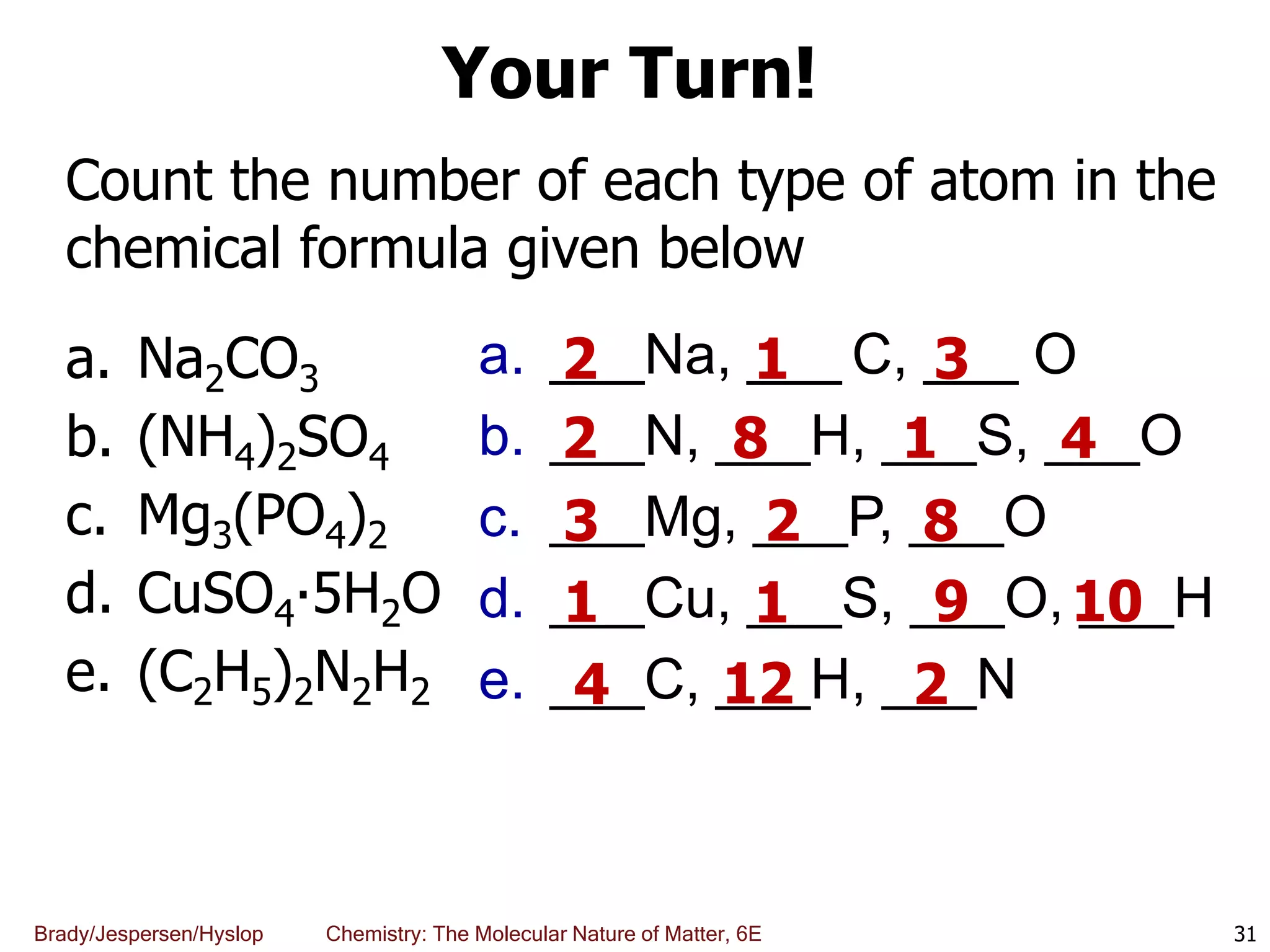
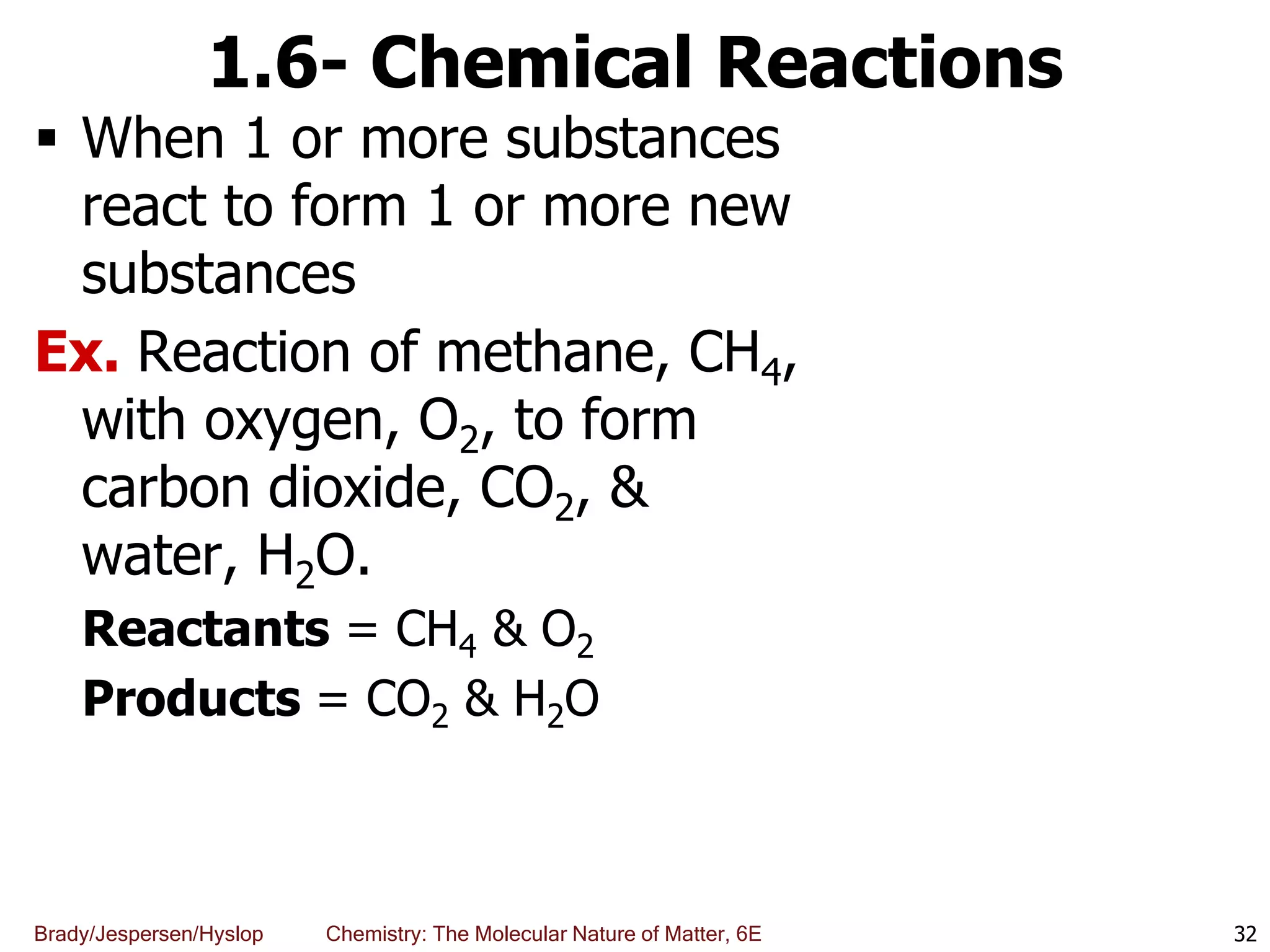
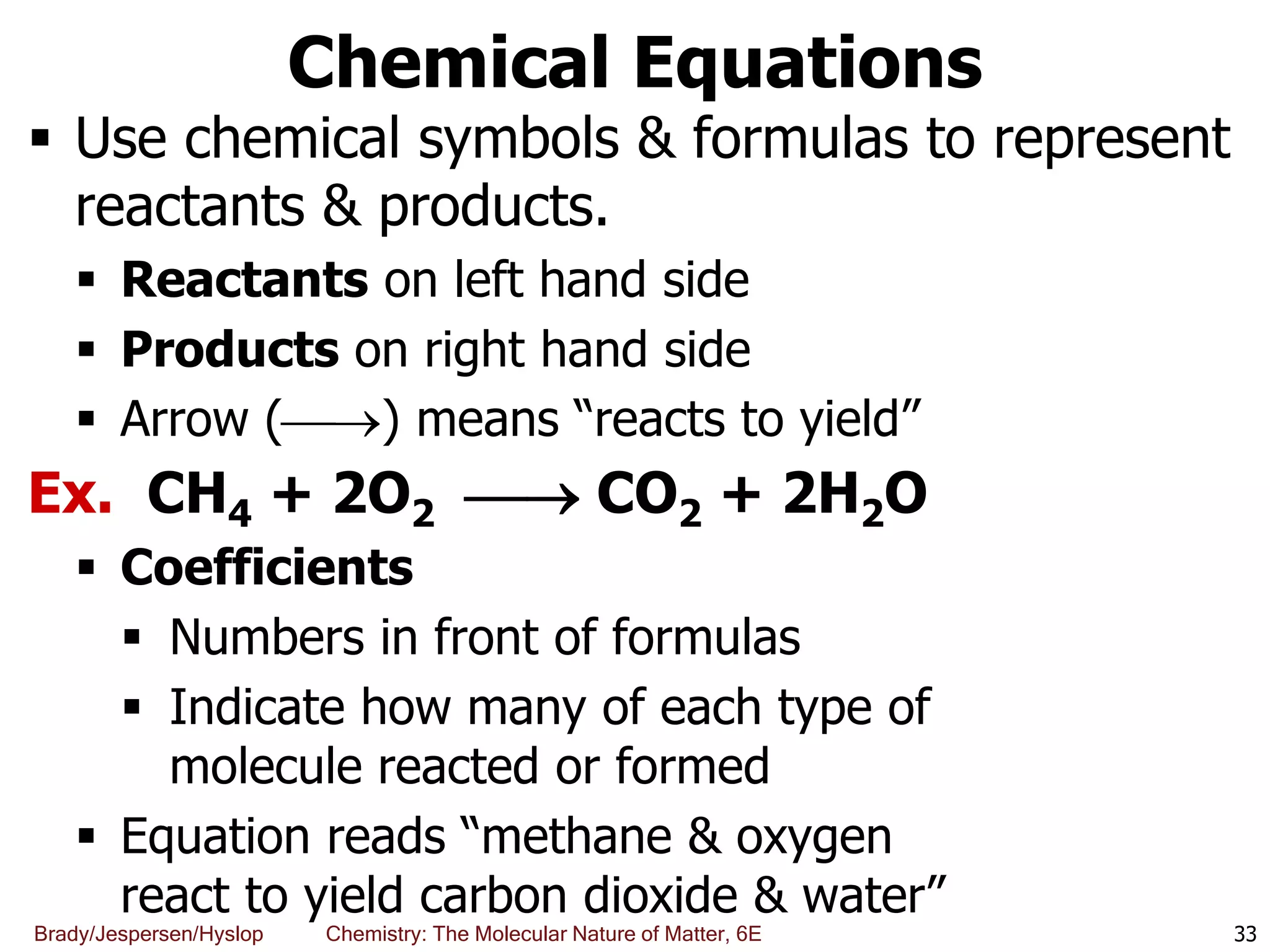
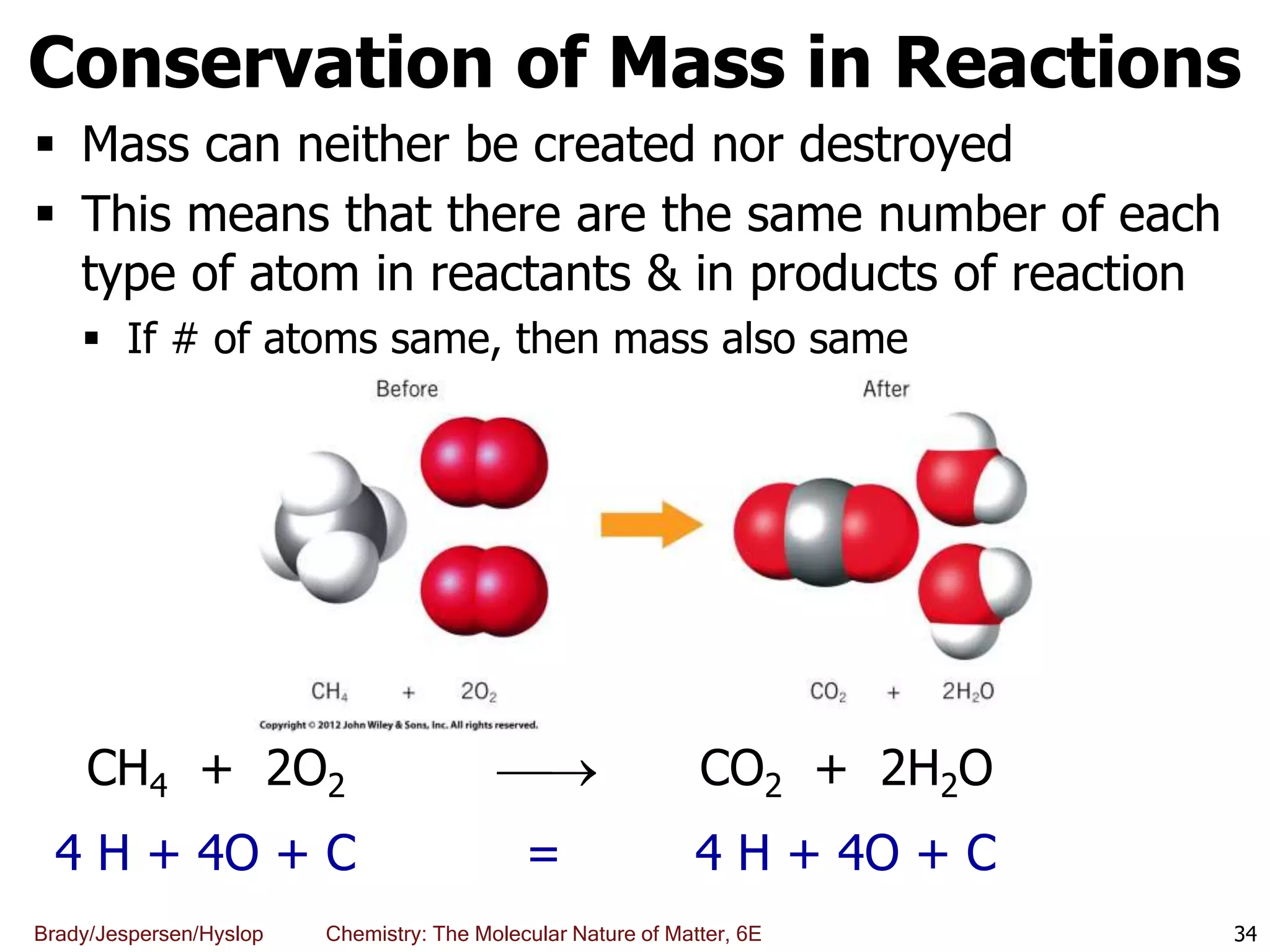
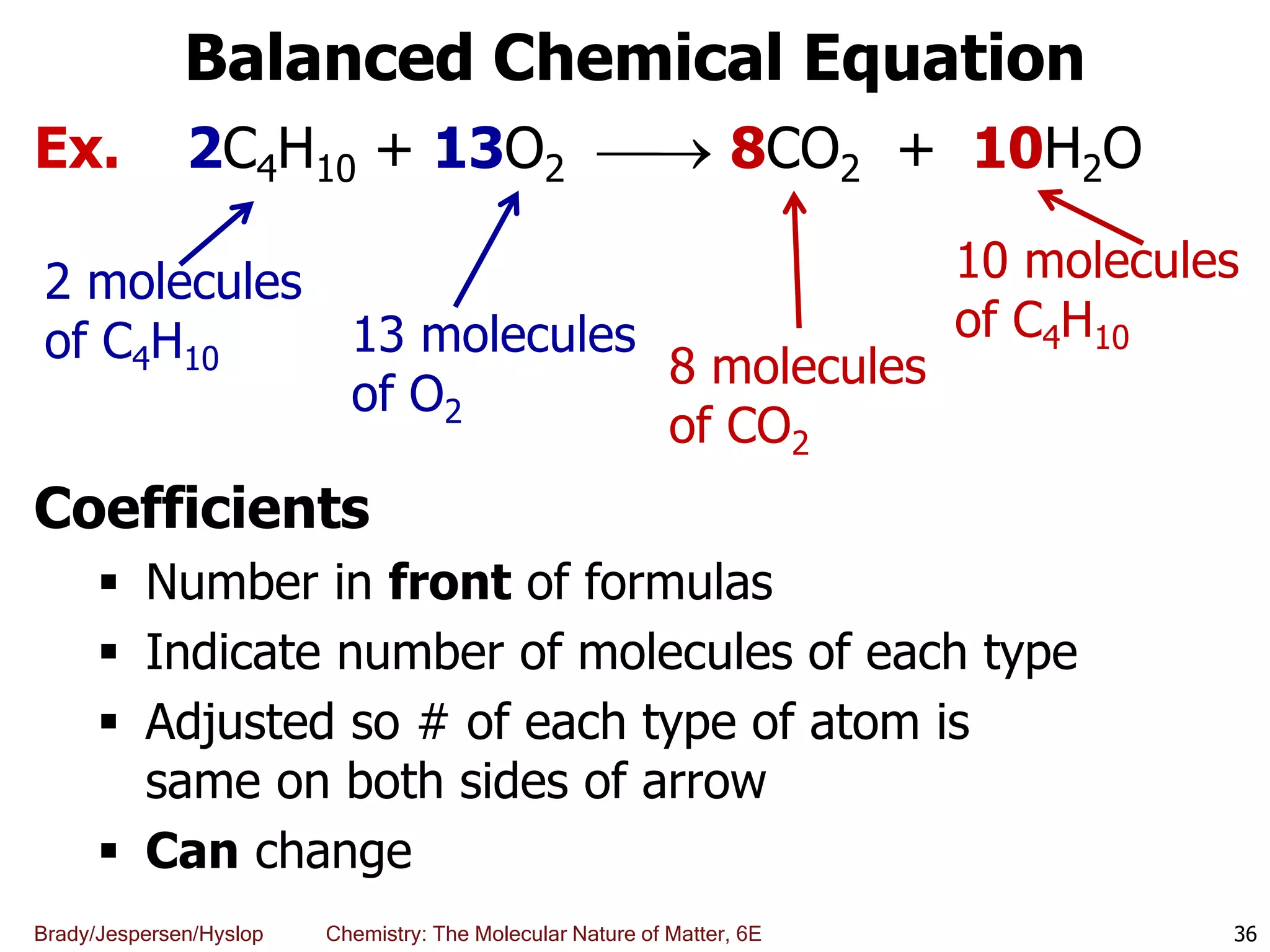
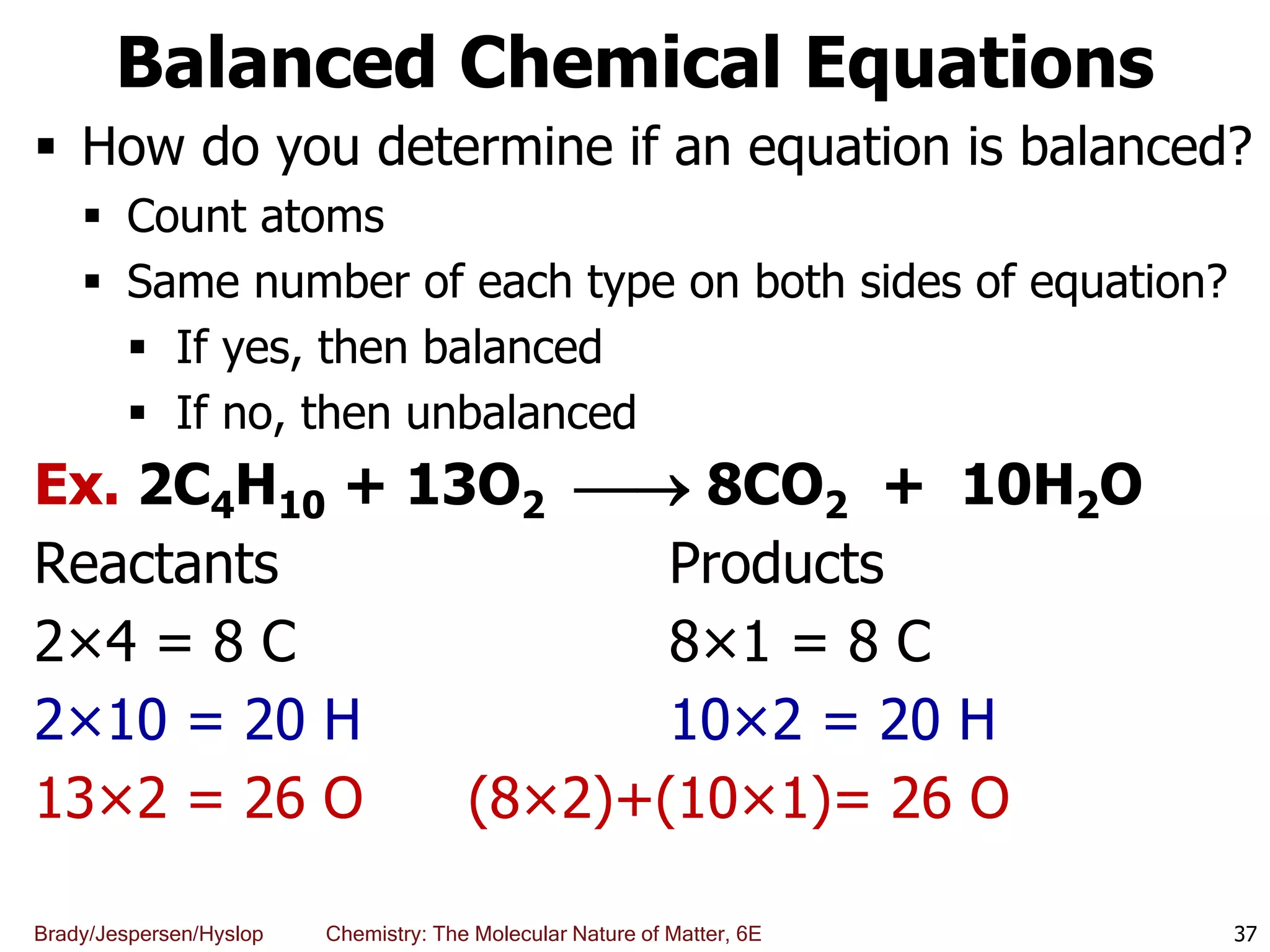
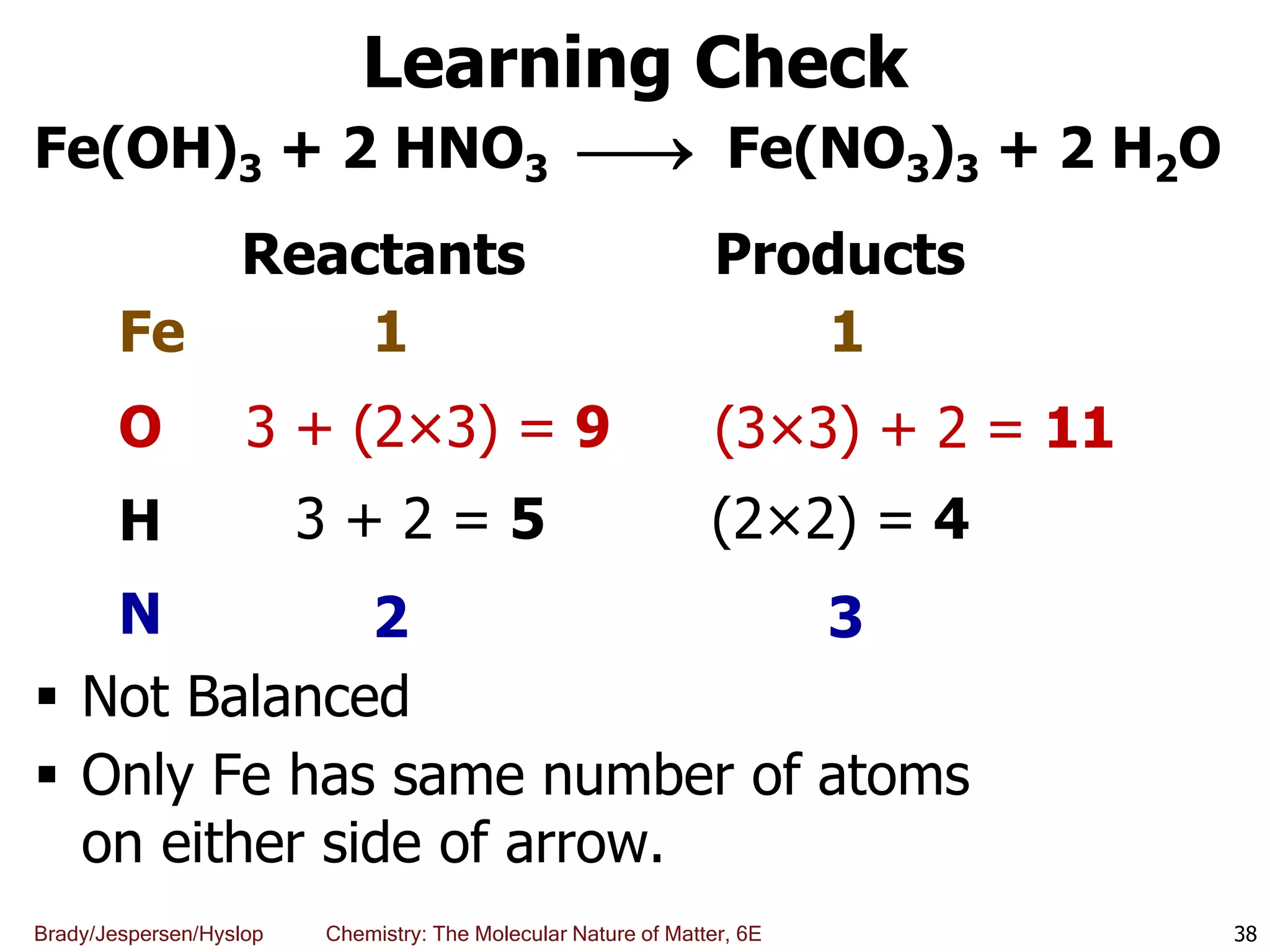
The document discusses key concepts in chemistry including the scientific method, atomic theory, and the classification of matter. It explains that chemistry uses the scientific method to study matter and its transformations. Matter is anything that has mass and takes up space, and can be classified as elements, compounds, or mixtures. Elements are substances made of only one type of atom, while compounds are made of two or more elements chemically bonded together. Mixtures can be either homogeneous, with consistent properties throughout, or heterogeneous, having distinct parts with different properties. Chemical and physical changes that matter undergoes are also described.