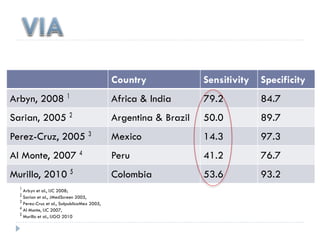
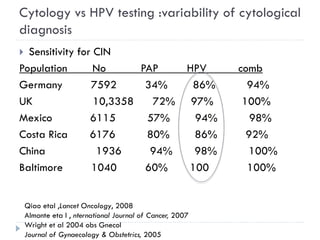
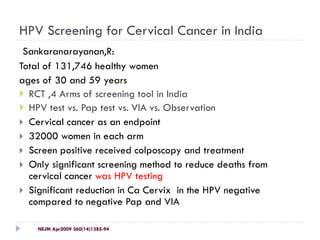
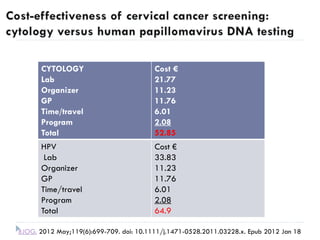
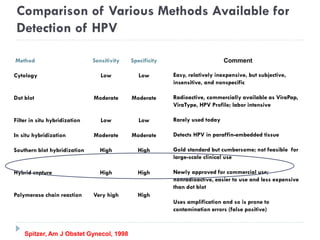
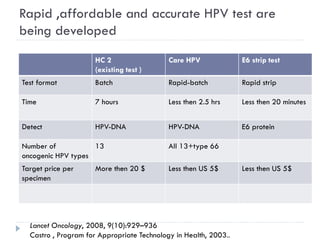
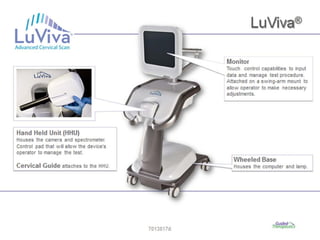
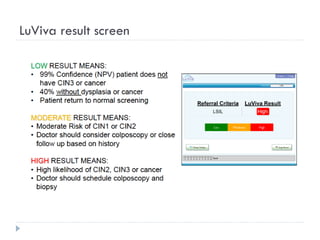
This document discusses cervical cancer screening and prevention. It provides the following key points:
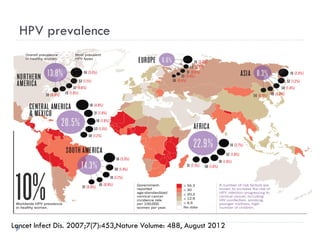
1. Cervical cancer is the 2nd most common cancer in women worldwide, with an estimated 530,000 new cases and 274,000 deaths annually, most occurring in developing countries.
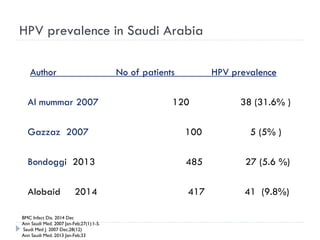
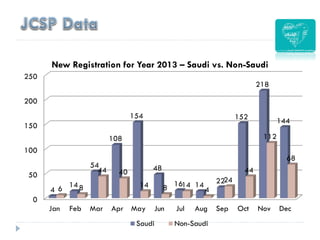
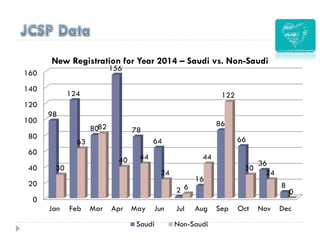
2. Incidence of cervical cancer is low in Saudi Arabia but it remains the 8th most common cancer in women, with 241 new cases and 84 deaths estimated annually.
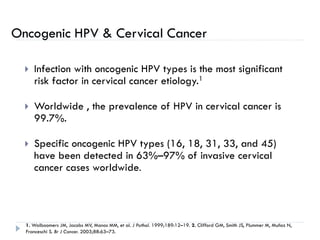
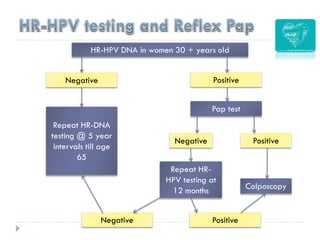
3. Infection with human papillomavirus (HPV) is the most significant risk factor for cervical cancer. Worldwide nearly 100% of cervical cancer cases are HPV-positive.
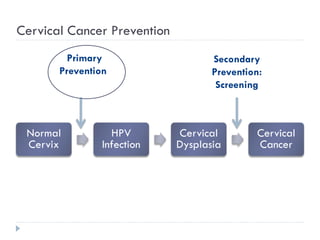
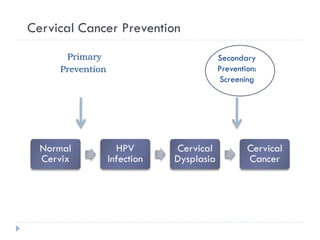
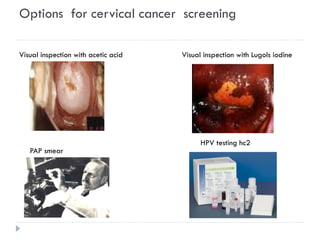
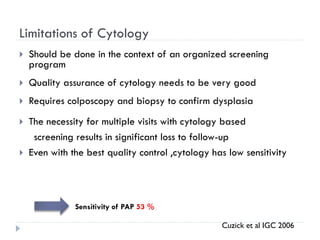
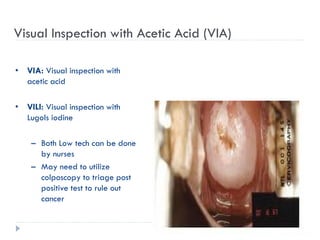
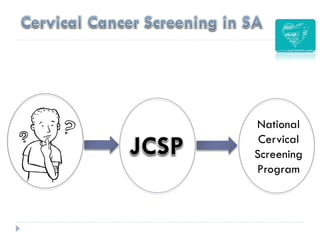
4. Screening is important for secondary cervical cancer prevention.