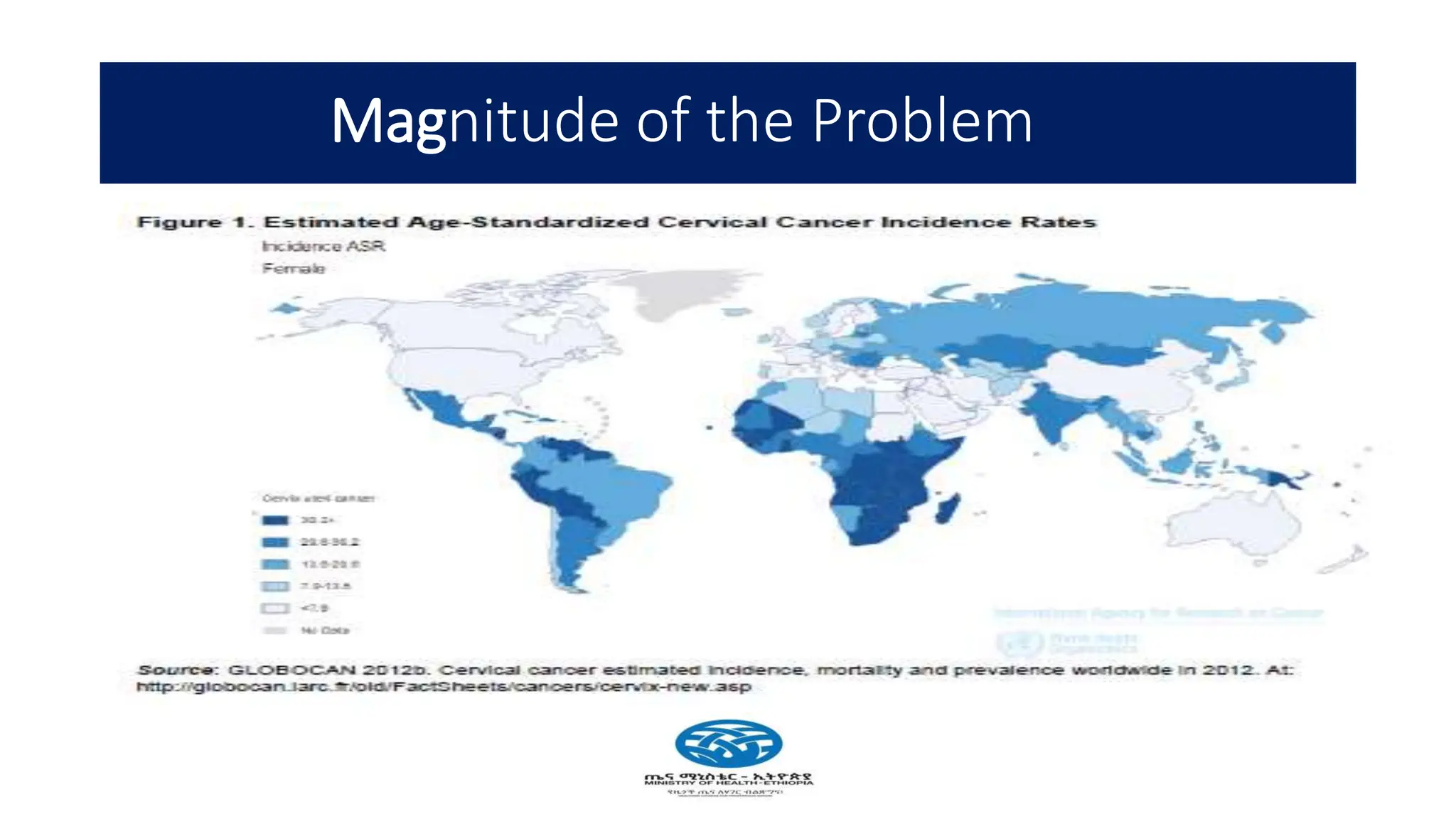
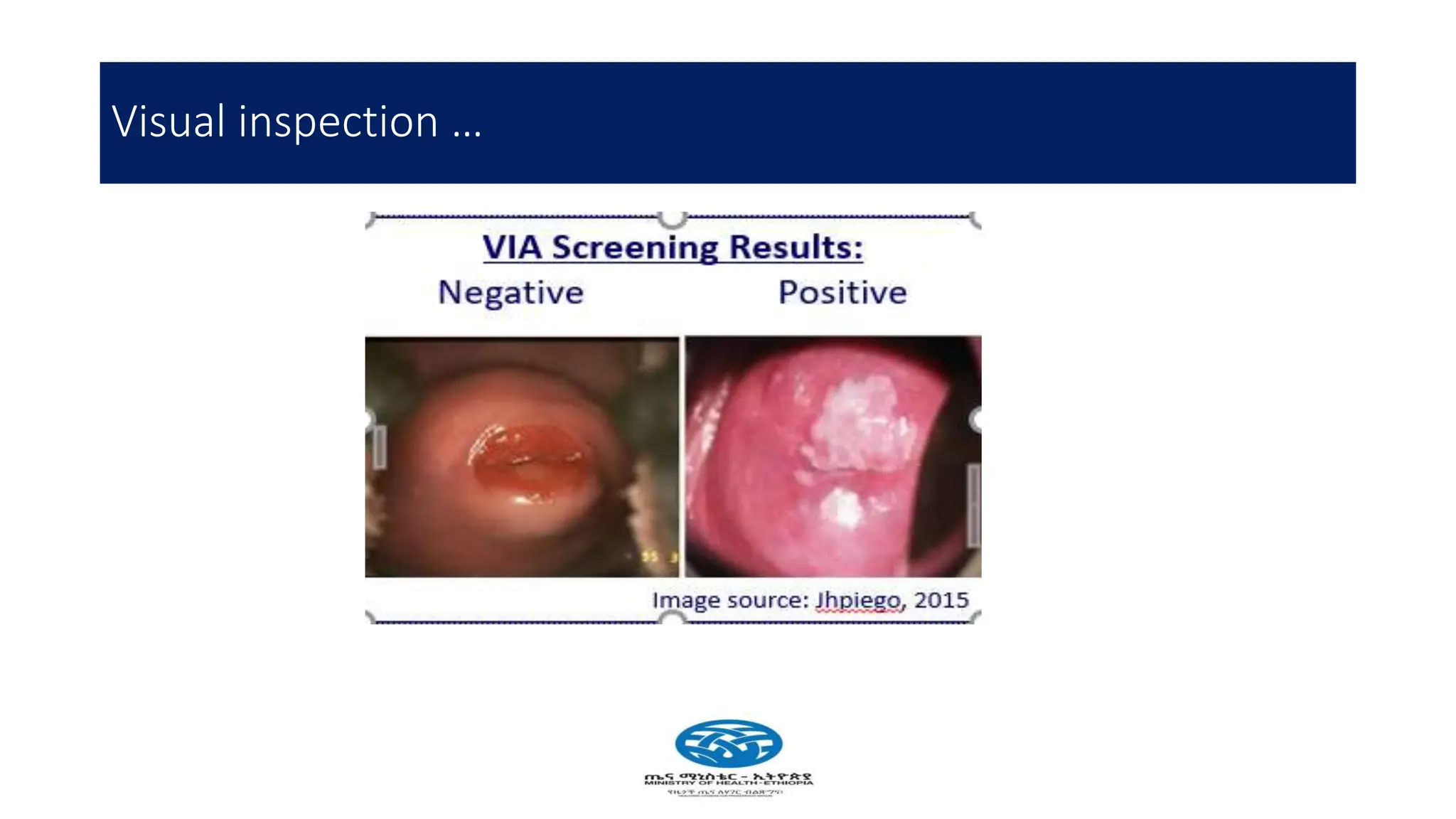
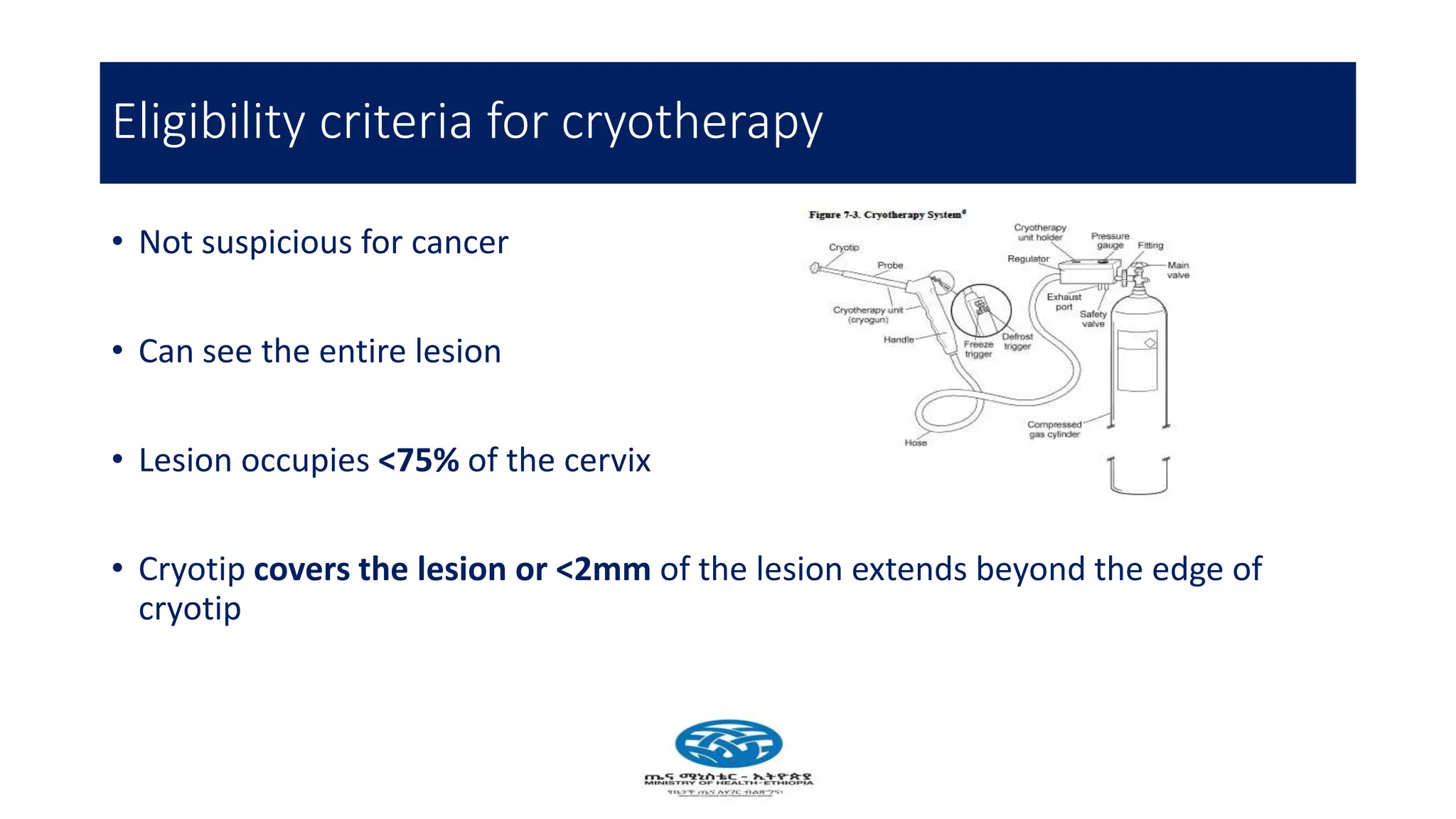
Cervical cancer poses a significant public health challenge, particularly in low and middle-income countries, with increasing mortality rates projected by 2040. Effective prevention and management strategies include HPV vaccination, regular screening for precancerous lesions, and immediate treatment options like cryotherapy and thermal ablation. The national screening strategy in Ethiopia focuses on a 'screen and treat' approach, prioritizing women aged 30-49 and integrating HIV testing for comprehensive care.