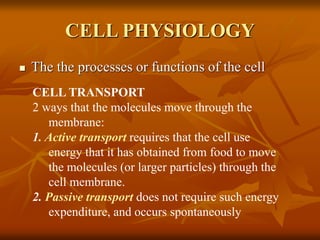
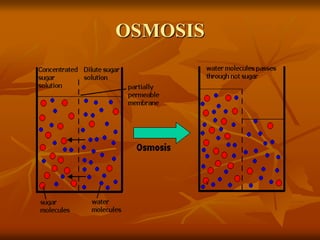
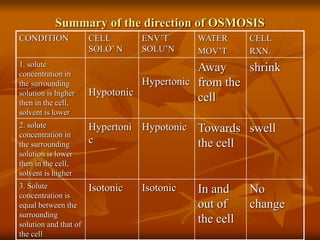
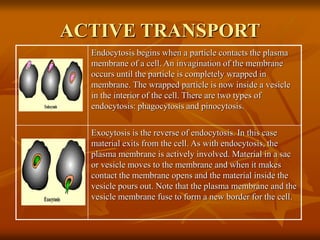
Cell physiology involves the processes and functions that occur within cells. There are two main types of cell transport: passive transport which does not require energy and occurs down a concentration gradient, and active transport which uses energy to move molecules against a concentration gradient. Osmosis is a type of passive transport where water molecules move through a semi-permeable membrane from an area of higher water concentration to lower concentration.