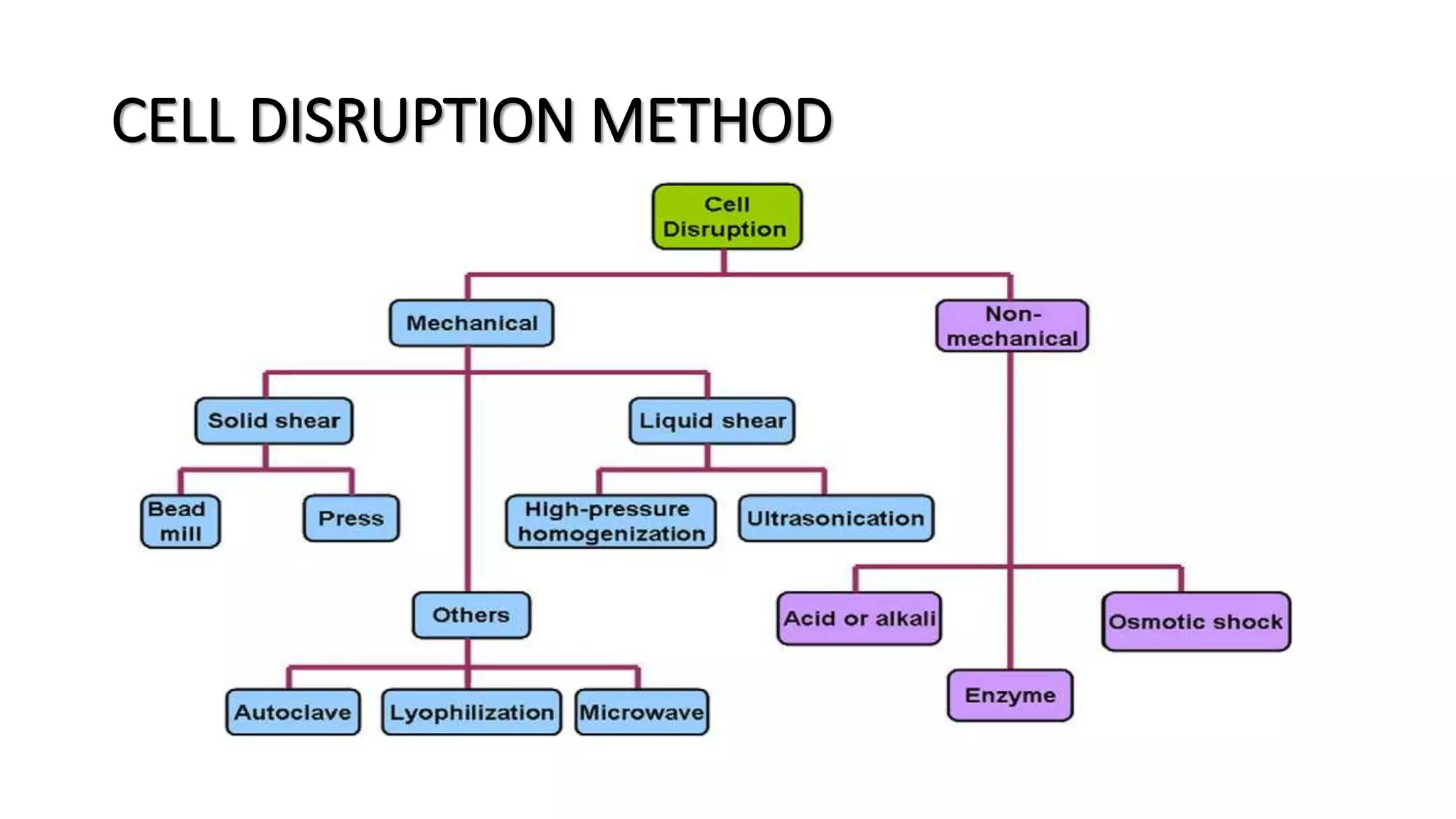
The document discusses osmotic shock as a non-mechanical physical method for cell disruption in biotechnical applications, highlighting its reliance on changes in salt concentration to induce cell lysis. It notes the challenges of using this method, including low efficiency, the need for enzymatic pre-treatment, high salt usage, and potential dilution of products, which can increase processing costs. Overall, while osmotic shock can effectively disrupt cells, its practical application is limited due to these drawbacks.