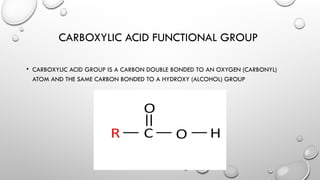
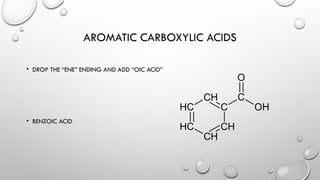
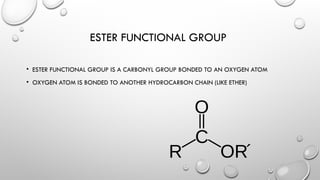
The document discusses carboxylic acids and esters in organic chemistry, describing their functional groups, properties, and naming conventions. Carboxylic acids are characterized by the carboxyl (COOH) group, while esters consist of a carbonyl group bonded to an oxygen atom and are formed through condensation reactions. The document also outlines the significance of these compounds in various applications, including their flavoring properties and boiling points.