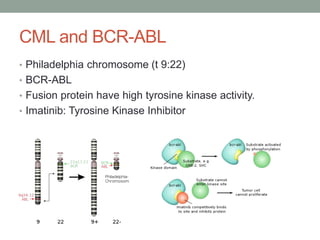
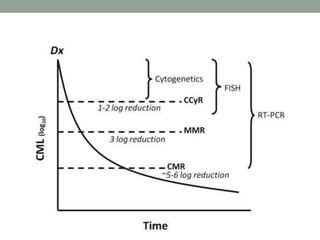
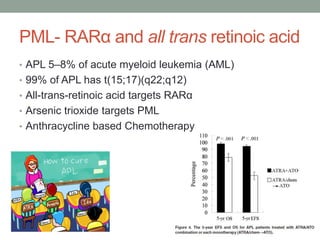
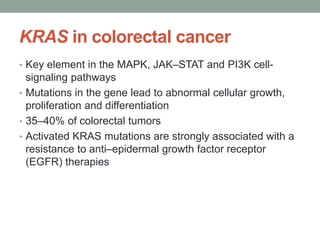
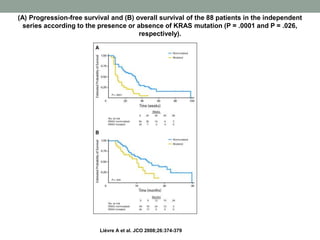
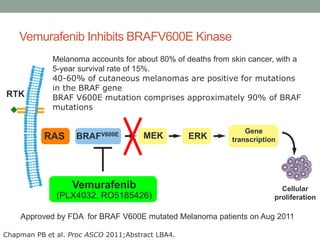
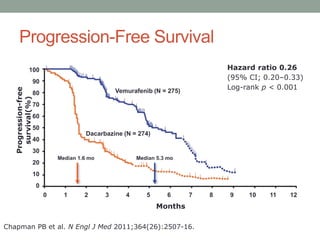
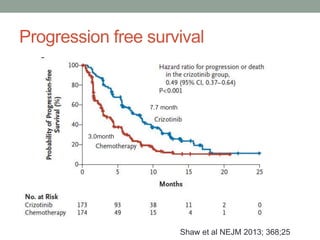
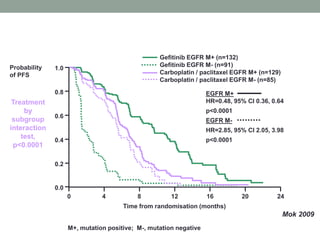
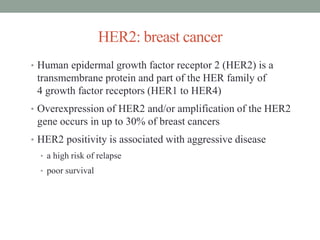
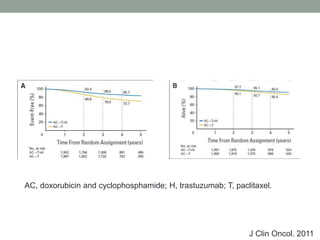
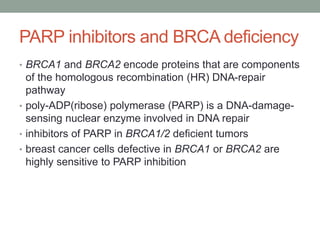
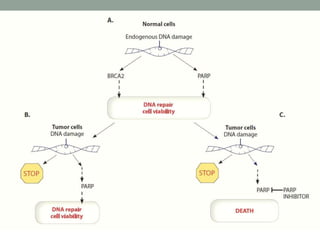
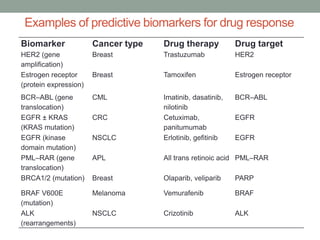
This document discusses the importance of cancer biomarkers for selecting effective targeted drug therapies. It provides examples of predictive biomarkers such as BCR-ABL for CML treated with tyrosine kinase inhibitors, EGFR mutations for NSCLC treated with EGFR inhibitors, and HER2 overexpression for breast cancer treated with trastuzumab. The use of predictive biomarkers can help personalize cancer treatment by identifying patients most likely to respond to a specific drug and avoid unnecessary toxicity for those who will not benefit.