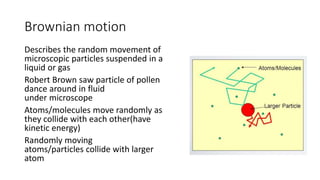
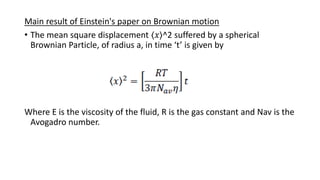
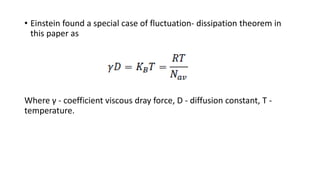
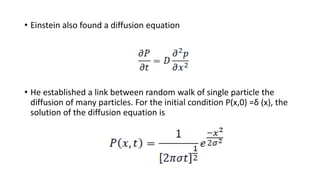
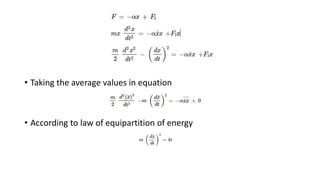
Brownian motion describes the random movement of microscopic particles suspended in a liquid or gas. Robert Brown first observed this random motion of pollen particles under a microscope. Einstein later provided a quantitative explanation for Brownian motion in 1905, relating the properties of Brownian motion and the diffusion constant. Langevin further developed a simpler theory of Brownian motion in 1908 based on assumptions of irregular motion and large numbers of collisions between particles.