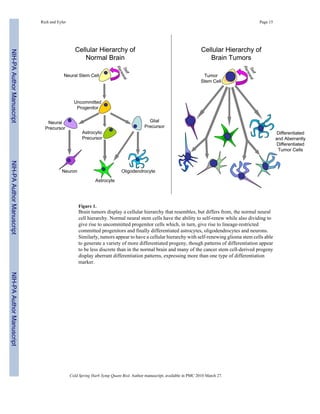
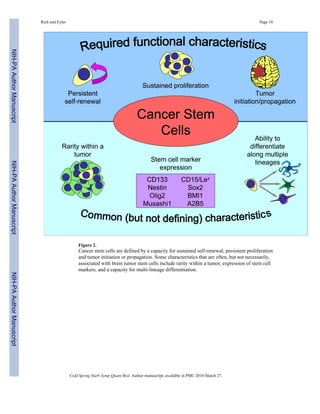
This document discusses cancer stem cells in brain tumors. It begins by providing background on brain tumors and their treatment challenges. It then discusses the cancer stem cell hypothesis, which proposes that tumors contain a cellular hierarchy with a subpopulation of stem-like cancer cells that can maintain and propagate the tumor. Studies have identified these cancer stem cells in various brain tumors through their ability to self-renew, proliferate, and initiate new tumors. However, there is still debate around this hypothesis due to inconsistencies across cancer types and lack of universal stem cell markers. The document focuses on research characterizing brain tumor stem cells and their roles in tumor biology and therapeutic resistance.






![Rich and Eyler Page 7
to consider CD133 as a validated prognostic indicator. The validation of other potential markers
remains less developed.
Brain Tumor Stem Cells in Therapeutic Resistance
Unfortunately, patients afflicted with malignant gliomas suffer nearly universal treatment
failure and death. As described above, surgical resection and cytotoxic modalities (radiation,
chemotherapy) remain the mainstay of brain tumor therapy [of note, anti-angiogenic therapy
in the form of the humanized neutralizing antibody against vascular endothelial growth factor
(VEGF) has shown initial promise]. The mechanisms through which brain tumors become
resistance to conventional therapy are poorly understood and are likely multi-factorial. We
examined a potential contribution of brain tumor stem cells to radiation resistance (Bao et al.
2006b). We found that ionizing radiation increased the relative frequency of tumor cells
expressing cancer stem cell markers in treated xenografts. The relative enrichment of these
cells was accompanied by maintained capacity for self renewal and tumor propagation whereas
matched non-stem cancer stem cells were more likely to die. Cancer stem cell enriched cultures
treated with radiation demonstrate a lower apoptotic fraction than non-stem cells in the same
conditions allowing for the outgrowth of the cancer stem cells. To elucidate a potential
mechanism, we studied the DNA damage checkpoint response in which a cascade of proteins
integrates signals from damage sensors to determine whether cells will initiate a cell cycle
arrest with DNA repair or undergo apoptosis. Cancer stem cells treated with radiation or radio-mimetics
displayed an increased activation of the DNA damage checkpoint response in
comparison to matched non-stem cancer cells. Although the activated proteins showed
variability between samples, some proteins appeared to be activated at baseline (e.g. Rad17)
as if the cancer stem cells were primed to respond to genotoxic stress, which may be an early
event in cancer initiation. The role of the DNA damage checkpoint response proved
contributory as a pharmacologic inhibitor of the checkpoint sensitized the cancer stem cells to
radiation. These results were supported by studies in genetically engineered medulloblastomas
(Hambardzumyan et al. 2008). Other researchers have also found that neurosphere forming
brain tumor cells are more resistant to chemotherapy than similar cells grown under
differentiating conditions (Liu et al. 2006). In sum, though these studies suggest that cancer
stem cells may contribute to the common therapeutic resistance of brain tumors and may be
targetable with pharmacologic approaches, it also seems unlikely that the full extent of
resistance in brain tumors derives from cancer stem cells.
Brain tumor stem cells in angiogenesis
Malignant gliomas are commonly angiogenic with vascular proliferation serving as an
informative histologic feature indicating a glioblastoma among the gliomas. Many growth
factors are secreted by malignant gliomas to stimulate and maintain neoangiogenic vasculature.
Targeted therapies have been developed against some of these pathways (reviewed in Jain et
al. 2007). Most clinical trials have demonstrated modest benefits to these agents, but a potential
clinical efficacy has been seen in several trials of bevacizumab (Avastin), a neutralizing
antibody against VEGF (Vredenburgh et al. 2006; Vredenburgh et al. 2007). Interestingly, the
activity of low molecular weight inhibitors against the VEGF receptors have been more modest
in clinical trial suggesting that the same molecular pathway may be targeted by different agents
with different outcomes. During our studies of brain tumor stem cells, we noted that cancer
stem cells form highly angiogenic tumors in comparison to the uncommon tumors that we
detect in propagation studies with cancer stem cell depleted cultures (Bao et al. 2006a). We
found that conditioned media from cancer stem cells strongly induced endothelial cell
migration, proliferation, and tube formation in contrast to non-stem cancer cell conditioned
media. Characterization of angiogenic proteins in the conditioned media revealed a consistent
upregulation of VEGF. We were able to specifically block the effects of cancer stem cell
Cold Spring Harb Symp Quant Biol. Author manuscript; available in PMC 2010 March 27.
NIH-PA Author Manuscript NIH-PA Author Manuscript NIH-PA Author Manuscript](https://image.slidesharecdn.com/braincancer-141201161444-conversion-gate01/85/Brain-cancer-7-320.jpg)


![Rich and Eyler Page 10
the aggressive investigation of new areas of research. Our studies and those of other laboratories
have suggested that brain tumor stem cells contribute to therapeutic resistance, tumor
angiogenesis, and invasion. Further characterization of this cellular fraction may guide the
development of biomarkers, imaging modalities, and treatments that will hopefully be more
effective. However, this field is immature and the progress forward will likely be made with
stumbles and errors but will be a learning process. The current challenge in deriving and
maintaining brain tumor stem cells is a major limitation in the field as most laboratories do not
have access to viable clinical specimens and animal resources. However, it is important to find
ways to adapt these techniques for widespread use since there is currently insufficient evidence
that established cell lines maintained for long periods in serum are useful in cancer stem cell
studies. Cell culture – particularly long term cell culture in medium containing serum – is well
recognized to induce genetic changes that were not present in the original tumor, limiting the
utility of cell lines in modeling the original disease. The development of validated brain tumor
models that can be shared in the field would be an important step forward. In addition, the
functional assays for all brain tumor stem cell studies must be standardized with current use
of serial neurosphere formation as a surrogate for self renewal and tumor propagation.
Available markers for brain tumor stem cells are imperfect and cannot be definitively linked
to a stem cell phenotype, supporting an urgent need for improved markers. As brain tumors
are likely heterogeneous diseases, universal marker immunophenotypes may not be identifiable
but markers may assist in subcategorizing tumors. Molecular regulators of brain tumor stem
cells may provide biomarkers, imaging targets, and therapeutic targets but it is likely that
molecules may be shared with normal somatic stem cells so their use may be complicated.
Regardless of the outcome, the recognition of the potential importance of the cancer stem cell
hypothesis has energized brain tumor research. The healthy debate between believers and
skeptics will almost certainly lead to completely unforeseen directions in the field of brain
tumor research and therapeutic development. In the end, we all hope to help those patients and
families who are afflicted by brain tumors.
Acknowledgments
Financial support was provided by the Childhood Brain Tumor Foundation, the Pediatric Brain Tumor Foundation of
the United States, Accelerate Brain Cancer Cure, Alexander and Margaret Stewart Trust, Brain Tumor Society,
Goldhirsh Foundation, Duke Comprehensive Cancer Center Stem Cell Initiative Grant, NIH grants NS047409,
NS054276, CA129958 and CA116659. J.R. is a Damon Runyon-Lilly Clinical Investigator supported by the Damon
Runyon Cancer Research Foundation.
REFERENCES
Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of
tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. U.S.A 2003;100:3983–3988. [PubMed:
12629218]
Bao S, Wu Q, Sathornsumetee S, Hao Y, Li Z, Hjelmeland AB, Shi Q, McLendon RE, Bigner DD, Rich
JN. Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth
factor. Cancer Res 2006a;66:7843–7848. [PubMed: 16912155]
Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma
stem cells promote radioresistance by preferential activation of the DNA damage response. Nature
2006b;444:756–760. [PubMed: 17051156]
Bao S, Wu Q, Li Z, Sathornsumetee S, Wang H, McLendon RE, Hjelmeland AB, Rich JN. Targeting
cancer stem cells through L1CAM suppresses glioma growth. Cancer Res 2008;68:6043–6048.
[PubMed: 18676824]
Bar EE, Chaudhry A, Lin A, Fan X, Schreck K, Matsui W, Piccirillo S, Vescovi AL, DiMeco F, Olivi
A, Eberhart CG. Cyclopamine-mediated hedgehog pathway inhibition depletes stem-like cancer cells
in glioblastoma. Stem Cells 2007;25:2524–2533. [PubMed: 17628016]
Cold Spring Harb Symp Quant Biol. Author manuscript; available in PMC 2010 March 27.
NIH-PA Author Manuscript NIH-PA Author Manuscript NIH-PA Author Manuscript](https://image.slidesharecdn.com/braincancer-141201161444-conversion-gate01/85/Brain-cancer-10-320.jpg)
![Rich and Eyler Page 11
Beier D, Hau P, Proescholdt M, Lohmeier A, Wischhusen J, Oefner PJ, Aigner L, Brawanski A, Bogdahn
U, Beier CP. CD133(+) and CD133(−) glioblastoma-derived cancer stem cells show differential
growth characteristics and molecular profiles. Cancer Res 2007;67:4010–4015. [PubMed: 17483311]
Bidlingmaier S, Zhu X, Liu B. The utility and limitations of glycosylated human CD133 epitopes in
defining cancer stem cells. J. Mol. Med 2008;86:1025–1032. [PubMed: 18535813]
Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a
primitive hematopoietic cell. Nature Med 1997;3:730–737. [PubMed: 9212098]
Bruggeman SW, Hulsman D, Tanger E, Buckle T, Blom M, Zevenhoven J, van Tellingen O, van and
Lohuizen M. Bmi1 controls tumor development in an Ink4a/Arf-independent manner in a mouse model
for glioma. Cancer Cell 2007;12:328–341. [PubMed: 17936558]
Bühring HJ, Seiffert M, Marxer A, Weiss B, Faul C, Kanz L, Brugger W. AC133 antigen expression is
not restricted to acute myeloid leukemia blasts but is also found on acute lymphoid leukemia blasts
and on a subset of CD34+ B-cell precursors. Blood 1999;94:832–833. [PubMed: 10438201]
Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, Oh EY, Gaber MW, Finklestein D,
Allen M, Frank A, Bayazitov IT, Zakharenko SS, Gajjar A, Davidoff A, Gilbertson RJ. A perivascular
niche for brain tumor stem cells. Cancer Cell 2007;11:69–82. [PubMed: 17222791]
Christensen K, Schrøder HD, Kristensen BW. CD133 identifies perivascular niches in grade II–IV
astrocytomas. J. Neurooncol 2008;90:157–170. [PubMed: 18612800]
Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CH, Jones DL, Visvader J, Weissman IL, Wahl GM.
Cancer stem cells--perspectives on current status and future directions: AACR Workshop on cancer
stem cells. Cancer Res 2006;66:9339–9344. [PubMed: 16990346]
Clement V, Sanchez P, de Tribolet N, Radovanovic I, Ruiz i Altaba A. HEDGEHOG-GLI1 signaling
regulates human glioma growth, cancer stem cell self-renewal, and tumorigenicity. Curr. Biol
2007;17:165–172. [PubMed: 17196391]
Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human
glioblastoma genes and core pathways. Nature 2008;455:1061–1068. [PubMed: 18772890]
Dalerba P, Dylla SJ, Park IK, Liu R, Wang X, Cho RW, Hoey T, Gurney A, Huang EH, Simeone DM,
Shelton AA, Parmiani G, Castelli C, Clarke MF. Phenotypic characterization of human colorectal
cancer stem cells. Proc. Natl. Acad. Sci. U.S.A 2007;104:10158–10163. [PubMed: 17548814]
Du Z, Jia D, Liu S, Wang F, Li G, Zhang Y, Cao X, Ling EA, Hao A. Oct4 is expressed in human gliomas
and promotes colony formation in glioma cells. Glia. 2008 [Epub ahead of print].
Eyler CE, Foo WC, Lafiura KM, McLendon RE, Hjelmeland AB, Rich JN. Brain Cancer Stem Cells
Display Preferential Sensitivity to Akt Inhibition. Stem Cells. 2008 [Epub ahead of print].
Fan X, Matsui W, Khaki L, Stearns D, Chun J, Li YM, Eberhart CG. Notch pathway inhibition depletes
stem-like cells and blocks engraftment in embryonal brain tumors. Cancer Res 2006;66:7445–7452.
[PubMed: 16885340]
Fulci G, Ishii N, Maurici D, Gernert K, Hainaut P, Kaur B, Van Meir EG. Initiation of human astrocytoma
by clonal evolution of cells with progressive loss of p53 functions in a patient with a 283H TP53
germline mutation: evidence for a precursor lesion. Cancer Res 2002;62:2897–2906. [PubMed:
12019170]
Furnari FB, Fenton T, Bachoo RM, Mukasa A, Stommel JM, Stegh A, Hahn WC, Ligon KL, Louis DN,
Brennan C, Chin L, DePinho RA, Cavenee WK. Malignant astrocytic glioma: genetics, biology, and
paths to treatment. Genes Dev 2007;21:2683–2710. [PubMed: 17974913]
Galli R, Binda E, Orfanelli U, Cipelletti B, Gritti A, De Vitis S, Fiocco R, Foroni C, Dimeco F, Vescovi
A. Isolation and characterization of tumorigenic, stem-like neural precursors from human
glioblastoma. Cancer Res 2004;64:7011–7021. [PubMed: 15466194]
Gangemi RM, Griffero F, Marubbi D, Perera M, Capra MC, Malatesta P, Ravetti GL, Zona GL, Daga
A, Corte G. SOX2 Silencing in Glioblastoma Tumor Initiating Cells Causes Stop of Proliferation
and Loss of Tumorigenicity. Stem Cells. 2008 [Epub ahead of print].
Gilbertson RJ, Rich JN. Making a tumour's bed: glioblastoma stem cells and the vascular niche. Nat. Rev.
Cancer 2007;10:733–736. [PubMed: 17882276]
Godlewski J, Nowicki MO, Bronisz A, Williams S, Otsuki A, Nuovo G, Raychaudhury A, Newton HB,
Chiocca EA, Lawler S. Targeting of the Bmi-1 oncogene/stem cell renewal factor by microRNA-128
Cold Spring Harb Symp Quant Biol. Author manuscript; available in PMC 2010 March 27.
NIH-PA Author Manuscript NIH-PA Author Manuscript NIH-PA Author Manuscript](https://image.slidesharecdn.com/braincancer-141201161444-conversion-gate01/85/Brain-cancer-11-320.jpg)
![Rich and Eyler Page 12
inhibits glioma proliferation and self-renewal. Cancer Res 2008;22:9125–9130. [PubMed:
19010882]
Hambardzumyan D, Becher OJ, Rosenblum MK, Pandolfi PP, Manova-Todorova K, Holland EC. PI3K
pathway regulates survival of cancer stem cells residing in the perivascular niche following radiation
in medulloblastoma in vivo. Genes Dev 2008;22:436–448. [PubMed: 18281460]
Hemmati HD, Nakano I, Lazareff JA, Masterman-Smith M, Geschwind DH, Bronner-Fraser M,
Kornblum HI. Cancerous stem cells can arise from pediatric brain tumors. Proc. Natl. Acad. Sci.
U.S.A 2003;100:15178–15183. [PubMed: 14645703]
Hope KJ, Jin L, Dick JE. Acute myeloid leukemia originates from a hierarchy of leukemic stem cell
classes that differ in self-renewal capacity. Nat. Immunol 2004;5:738–743. [PubMed: 15170211]
Horn PA, Tesch H, Staib P, Kube D, Diehl V, Voliotis D. Expression of AC133, a novel hematopoietic
precursor antigen, on acute myeloid leukemia cells. Blood 1999;93:1435–1437. [PubMed:
10075457]
Howard BM, Boockvar JA. Stem Cell Marker CD133 Expression Predicts Outcome in Glioma Patients.
Neurosurgery 2008;62:N8.
Ignatova TN, Kukekov VG, Laywell ED, Suslov ON, Vrionis FD, Steindler DA. Human cortical glial
tumors contain neural stem-like cells expressing astroglial and neuronal markers in vitro. Glia
2002;39:193–206. [PubMed: 12203386]
Jain RK, di Tomaso E, Duda DG, Loeffler JS, Sorensen AG, Batchelor TT. Angiogenesis in brain
tumours. Nat. Rev. Neurosci 2007;8:610–622. [PubMed: 17643088]
Jaksch M, Múnera J, Bajpai R, Terskikh A, Oshima RG. Cell cycle-dependent variation of a CD133
epitope in human embryonic stem cell, colon cancer, and melanoma cell lines. Cancer Res
2008;68:7882–7886. [PubMed: 18829544]
Kelly PN, Dakic A, Adams JM, Nutt SL, Strasser A. Tumor growth need not be driven by rare cancer
stem cells. Science 2007;20:337. 317(5836). [PubMed: 17641192]
Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri
MA, Dick JE. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice.
Nature 1994;367:645–648. [PubMed: 7509044]
Lee J, Kotliarova S, Kotliarov Y, Li A, Su Q, Donin NM, Pastorino S, Purow BW, Christopher N, Zhang
W, Park JK, Fine HA. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more
closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines.
Cancer Cell 2006;9:391–403. [PubMed: 16697959]
Lee J, Son MJ, Woolard K, Donin NM, Li A, Cheng CH, Kotliarova S, Kotliarov Y, Walling J, Ahn S,
Kim M, Totonchy M, Cusack T, Ene C, Ma H, Su Q, Zenklusen JC, Zhang W, Maric D, Fine FA.
Epigenetic-mediated dysfunction of the bone morphogenetic protein pathway inhibits differentiation
of glioblastoma-initiating cells. Cancer Cell 2008;13:69–80. [PubMed: 18167341]
Lee ST, Jang JH, Min YH, Hahn JS, Ko YW. AC133 antigen as a prognostic factor in acute leukemia.
Leuk. Res 2001;25:757–767. [PubMed: 11489469]
Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, Wicha M, Clarke MF, Simeone DM.
Identification of pancreatic cancer stem cells. Cancer Res 2007;67:1030–1037. [PubMed: 17283135]
Ligon KL, Huillard E, Mehta S, Kesari S, Liu H, Alberta JA, Bachoo RM, Kane M, Louis DN, Depinho
RA, Anderson DJ, Stiles CD, Rowitch DH. Olig2-regulated lineage-restricted pathway controls
replication competence in neural stem cells and malignant glioma. Neuron 2007;53:503–517.
[PubMed: 17296553]
Liu G, Yuan X, Zeng Z, Tunici P, Ng H, Abdulkadir IR, Lu L, Irvin D, Black KL, Yu JS. Analysis of
gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol. Cancer
2006;5:67. [PubMed: 17140455]
Ma YH, Mentlein R, Knerlich F, Kruse ML, Mehdorn HM, Held-Feindt J. Expression of stem cell markers
in human astrocytomas of different WHO grades. J. Neurooncol 2008;86:31–45. [PubMed:
17611714]
Miraglia S, Godfrey W, Yin AH, Atkins K, Warnke R, Holden JT, Bray RA, Waller EK, Buck DW. A
novel five-transmembrane hematopoietic stem cell antigen: isolation, characterization, and molecular
cloning. Blood 1997;90:5013–5021. [PubMed: 9389721]
Cold Spring Harb Symp Quant Biol. Author manuscript; available in PMC 2010 March 27.
NIH-PA Author Manuscript NIH-PA Author Manuscript NIH-PA Author Manuscript](https://image.slidesharecdn.com/braincancer-141201161444-conversion-gate01/85/Brain-cancer-12-320.jpg)
![Rich and Eyler Page 13
Nathan C, Gold B, Lin G, Stegman M, de Carvalho LP, Vandal O, Venugopal A, Bryk R. A philosophy
of anti-infectives as a guide in the search for new drugs for tuberculosis. Tuberculosis (Edinb)
2008;1:S25–S33. [PubMed: 18762150]
O'Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour
growth in immunodeficient mice. Nature 2007;445:106–110. [PubMed: 17122772]
Panchision DM, Chen HL, Pistollato F, Papini D, Ni HT, Hawley TS. Optimized flow cytometric analysis
of central nervous system tissue reveals novel functional relationships among cells expressing
CD133, CD15, and CD24. Stem Cells 2007;25:1560–1570. [PubMed: 17332513]
Piccirillo SG, Reynolds BA, Zanetti N, Lamorte G, Binda E, Broggi G, Brem H, Olivi A, Dimeco F,
Vescovi AL. Bone morphogenetic proteins inhibit the tumorigenic potential of human brain tumour-initiating
cells. Nature 2006;444:761–765. [PubMed: 17151667]
Pope WB, Lai A, Nghiemphu P, Mischel P, Cloughesy TF. MRI in patients with high-grade gliomas
treated with bevacizumab and chemotherapy. Neurology 2006;8:1258–1260. [PubMed: 16636248]
Quintana E, Shackleton M, Sabel MS, Fullen DR, Johnson TM, Morrison SJ. Efficient tumour formation
by single human melanoma cells. Nature 2008;456:593–598. [PubMed: 19052619]
Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature
2001;414:105–111. [PubMed: 11689955]
Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, De Maria R. Identification
and expansion of human colon-cancer initiating cells. Nature 2007;445:111–115. [PubMed:
17122771]
Rietze RL, Valcanis H, Brooker GF, Thomas T, Voss AK, Bartlett PF. Purification of a pluripotent neural
stem cell from the adult mouse brain. Nature 2001;412:736–739. [PubMed: 11507641]
Sanai N, Tramontin AD, Quiñones-Hinojosa A, Barbaro NM, Gupta N, Kunwar S, Lawton MT,
McDermott MW, Parsa AT, Manuel-García Verdugo J, Berger MS, Alvarez-Buylla A. Unique
astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature
2004;427:740–744. [PubMed: 14973487]
Schüller U, Heine VM, Mao J, Kho AT, Dillon AK, Han YG, Huillard E, Sun T, Ligon AH, Qian Y, Ma
Q, Alvarez-Buylla A, McMahon AP, Rowitch DH, Ligon KL. Acquisition of granule neuron
precursor identity is a critical determinant of progenitor cell competence to form Shh-induced
medulloblastoma. Cancer Cell 2008;2:123–134.
Shu Q, Wong KK, Su JM, Adesina AM, Yu LT, Tsang YT, Antalffy BC, Baxter P, Perlaky L, Yang J,
Dauser RC, Chintagumpala M, Blaney SM, Lau CC, Li XN. Direct orthotopic transplantation of
fresh surgical specimen preserves CD133+ tumor cells in clinically relevant mouse models of
medulloblastoma and glioma. Stem Cells 2008;26:1414–1424. [PubMed: 18403755]
Singec I, Knoth R, Meyer RP, Maciaczyk J, Volk B, Nikkhah G, Frotscher M, Snyder EY. Defining the
actual sensitivity and specificity of the neurosphere assay in stem cell biology. Nat. Methods
2006;3:801–806. [PubMed: 16990812]
Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB. Identification of a cancer
stem cell in human brain tumors. Cancer Res 2003;63:5821–5828. [PubMed: 14522905]
Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks
PB. Identification of human brain tumour initiating cells. Nature 2004;432:396–401. [PubMed:
15549107]
Soeda A, Inagaki A, Oka N, Ikegame Y, Aoki H, Yoshimura S, Nakashima S, Kunisada T, Iwama T.
Epidermal growth factor plays a crucial role in mitogenic regulation of human brain tumor stem cells.
J. Biol. Chem 2008;283:10958–10966. [PubMed: 18292095]
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA,
Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D,
Cairncross JG, Eisenhauer E, Mirimanoff RO. European Organisation for Research and Treatment
of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials
Group. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med
2005;352:987–996. [PubMed: 15758009]
Tabu K, Sasai K, Kimura T, Wang L, Aoyanagi E, Kohsaka S, Tanino M, Nishihara H, Tanaka S. Promoter
hypomethylation regulates CD133 expression in human gliomas. Cell Res 2008;18:1037–1046.
[PubMed: 18679414]
Cold Spring Harb Symp Quant Biol. Author manuscript; available in PMC 2010 March 27.
NIH-PA Author Manuscript NIH-PA Author Manuscript NIH-PA Author Manuscript](https://image.slidesharecdn.com/braincancer-141201161444-conversion-gate01/85/Brain-cancer-13-320.jpg)
![Rich and Eyler Page 14
Taylor MD, Poppleton H, Fuller C, Su X, Liu Y, Jensen P, Magdaleno S, Dalton J, Calabrese C, Board
J, Macdonald T, Rutka J, Guha A, Gajjar A, Curran T, Gilbertson RJ. Radial glia cells are candidate
stem cells of ependymoma. Cancer Cell 2005;8:323–335. [PubMed: 16226707]
Thon N, Damianoff K, Hegermann J, Grau S, Krebs B, Schnell O, Tonn JC, Goldbrunner R. Presence of
pluripotent CD133(+) cells correlates with malignancy of gliomas. Mol. Cell. Neurosci. 2008 [Epub
ahead of print].
Uchida N, Buck DW, He D, Reitsma MJ, Masek M, Phan TV, Tsukamoto AS, Gage FH, Weissman IL.
Direct isolation of human central nervous system stem cells. Proc. Natl. Acad. Sci. U.S.A
2000;97:14720–14725. [PubMed: 11121071]
Vredenburgh JJ, Desjardins A, Herndon JE 2nd, Dowell JM, Reardon DA, Quinn JA, Rich JN,
Sathornsumetee S, Gururangan S, Wagner M, Bigner DD, Friedman AH, Friedman HS. Phase II trial
of bevacizumab and irinotecan in recurrent malignant glioma. Clin. Cancer Res 2007a;13:1253–1259.
[PubMed: 17317837]
Vredenburgh JJ, Desjardins A, Herndon JE 2nd, Marcello J, Reardon DA, Quinn JA, Rich JN,
Sathornsumetee S, Gururangan S, Sampson J, Wagner M, Bailey L, Bigner DD, Friedman AH,
Friedman HS. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J. Clin. Oncol
2007;25:4722–4729. [PubMed: 17947719]
Wang J, Wang H, Li Z, Wu Q, Lathia JD, McLendon RE, Hjelmeland AB, Rich JN. c-Myc is required
for maintenance of glioma cancer stem cells. PLoS ONE 2008;11:e3769. [PubMed: 19020659]
Weigmann A, Corbeil D, Hellwig A, Huttner WB. Prominin, a novel microvilli-specific polytopic
membrane protein of the apical surface of epithelial cells, is targeted to plasmalemmal protrusions
of non-epithelial cells. Proc. Natl. Acad. Sci U.S.A 1997;94:12425–12430. [PubMed: 9356465]
Wu M, Kwon HY, Rattis F, Blum J, Zhao C, Ashkenazi R, Jackson TL, Gaiano N, Oliver T, Reya T.
Imaging hematopoietic precursor division in real time. Cell Stem Cell 2007;5:541–554. [PubMed:
18345353]
Yang ZJ, Ellis T, Markant SL, Read TA, Kessler JD, Bourboulas M, Schüller U, Machold R, Fishell G,
Rowitch DH, Wainwright BJ, Wechsler-Reya RJ. Medulloblastoma can be initiated by deletion of
Patched in lineage-restricted progenitors or stem cells. Cancer Cell 2008b;2:135–145.
Yang Z, Chen Y, Lillo C, Chien J, Yu Z, Michaelides M, Klein M, Howes KA, Li Y, Kaminoh Y, Chen
H, Zhao C, Chen Y, Al-Sheikh YT, Karan G, Corbeil D, Escher P, Kamaya S, Li C, Johnson S,
Frederick JM, Zhao Y, Wang C, Cameron DJ, Huttner WB, Schorderet DF, Munier FL, Moore AT,
Birch DG, Baehr W, Hunt DM, Williams DS, Zhang K. Mutant prominin 1 found in patients with
macular degeneration disrupts photoreceptor disk morphogenesis in mice. J. Clin. Invest 2008a;
118:2908–2916. [PubMed: 18654668]
Yi JM, Tsai HC, Glöckner SC, Lin S, Ohm JE, Easwaran H, James CD, Costello JF, Riggins G, Eberhart
CG, Laterra J, Vescovi AL, Ahuja N, Herman JG, Schuebel KE, Baylin SB. Abnormal DNA
methylation of CD133 in colorectal and glioblastoma tumors. Cancer Res 2008;68:8094–8103.
[PubMed: 18829568]
Yin AH, Miraglia S, Zanjani ED, Almeida-Porada G, Ogawa M, Leary AG, Olweus J, Kearney J, Buck
DW. AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood
1997;90:5002–5012. [PubMed: 9389720]
Yuan X, Curtin J, Xiong Y, Liu G, Waschsmann-Hogiu S, Farkas DL, Black KL, Yu JS. Isolation of
cancer stem cells from adult glioblastoma multiforme. Oncogene 2004;23:9392–9400. [PubMed:
15558011]
Zeppernick F, Ahmadi R, Campos B, Dictus C, Helmke BM, Becker N, Lichter P, Unterberg A,
Radlwimmer B, Herold-Mende CC. Stem cell marker CD133 affects clinical outcome in glioma
patients. Clin. Cancer Res 2008;14:123–129. [PubMed: 18172261]
Zheng H, Ying H, Yan H, Kimmelman AC, Hiller DJ, Chen AJ, Perry SR, Tonon G, Chu GC, Ding Z,
Stommel JM, Dunn KL, Wiedemeyer R, You MJ, Brennan C, Wang YA, Ligon KL, Wong WH,
Chin L, DePinho RA. p53 and Pten control neural and glioma stem/progenitor cell renewal and
differentiation. Nature 2008;7216:1129–1133. [PubMed: 18948956]
Cold Spring Harb Symp Quant Biol. Author manuscript; available in PMC 2010 March 27.
NIH-PA Author Manuscript NIH-PA Author Manuscript NIH-PA Author Manuscript](https://image.slidesharecdn.com/braincancer-141201161444-conversion-gate01/85/Brain-cancer-14-320.jpg)