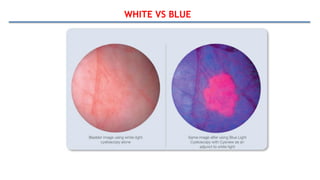
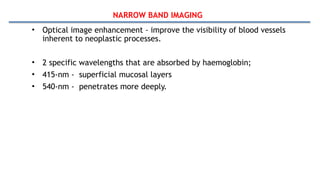
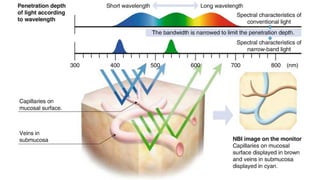
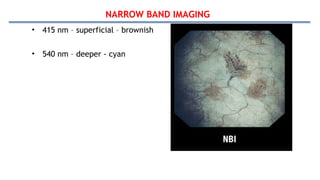
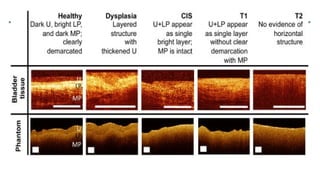
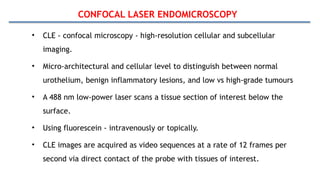
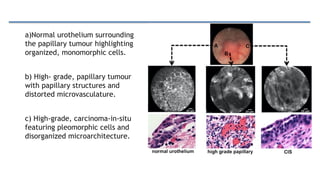
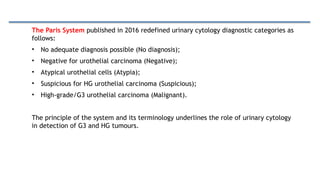
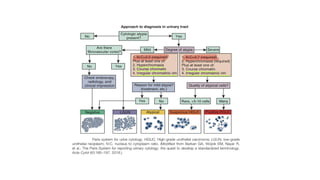
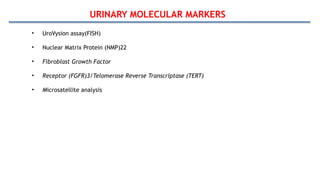
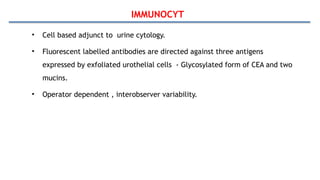
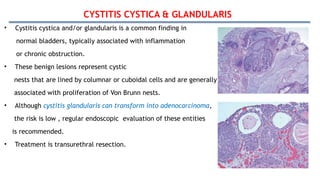
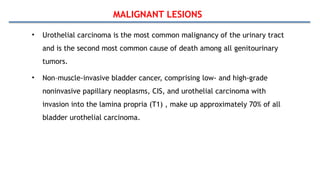
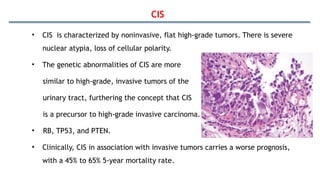
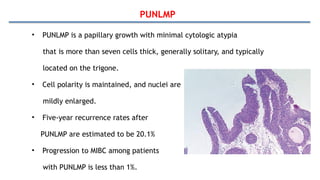
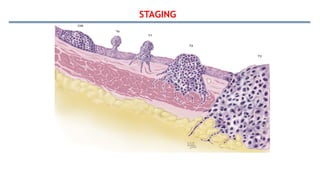
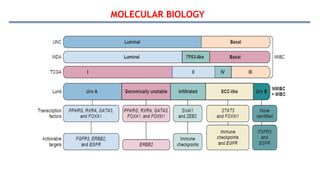
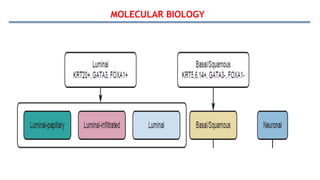
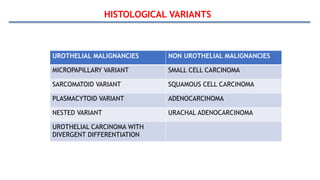
Bladder tumors, specifically bladder cancer, occur when abnormal cells grow uncontrollably in the bladder lining, forming a tumor. These tumors can be non-muscle invasive (staying within the bladder lining) or muscle-invasive (penetrating into the bladder muscle). The most common type of bladder cancer is urothelial carcinoma.
Key Points:
Types of Bladder Tumors:
The most common type is urothelial carcinoma, followed by squamous cell carcinoma and adenocarcinoma