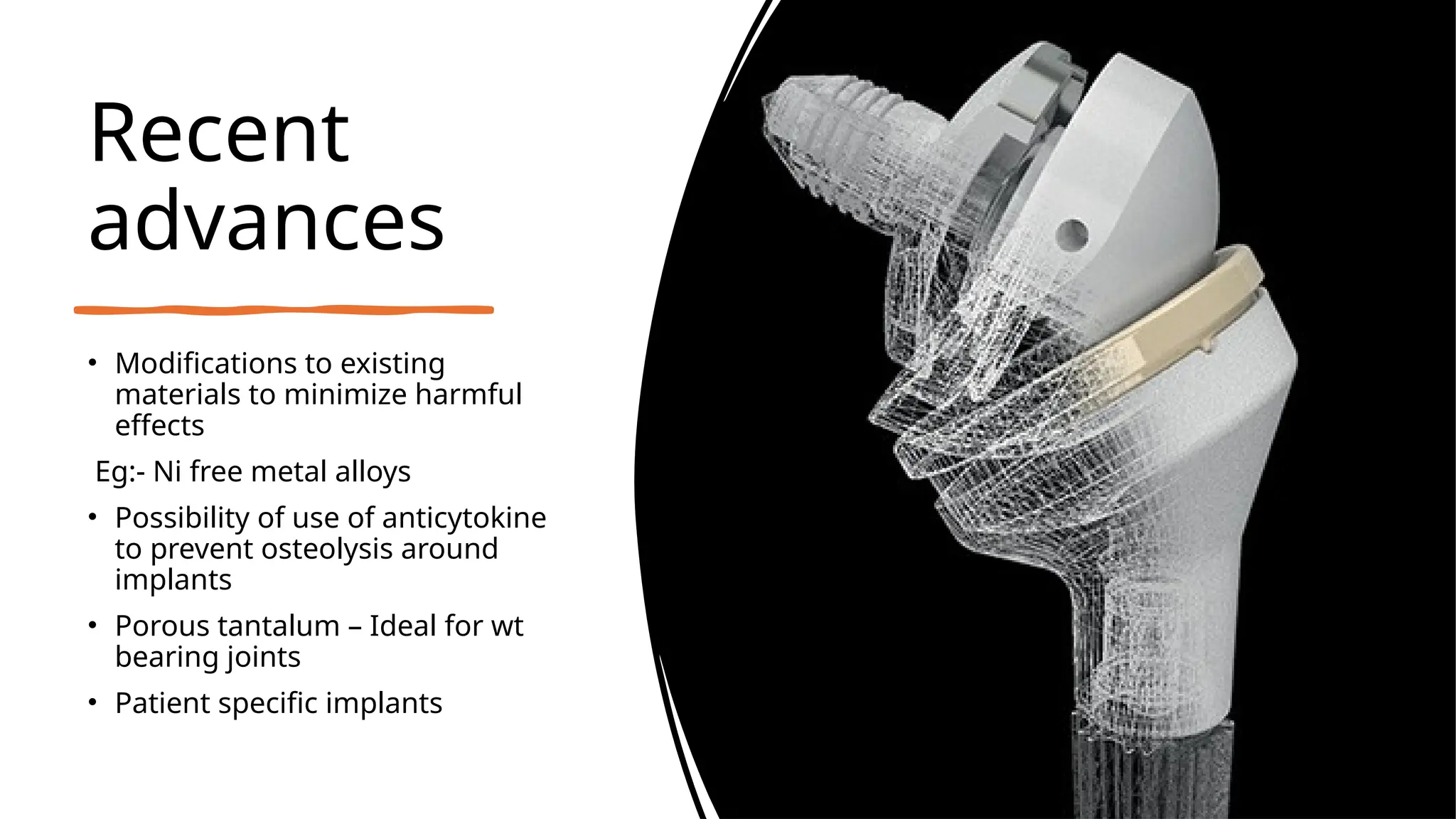
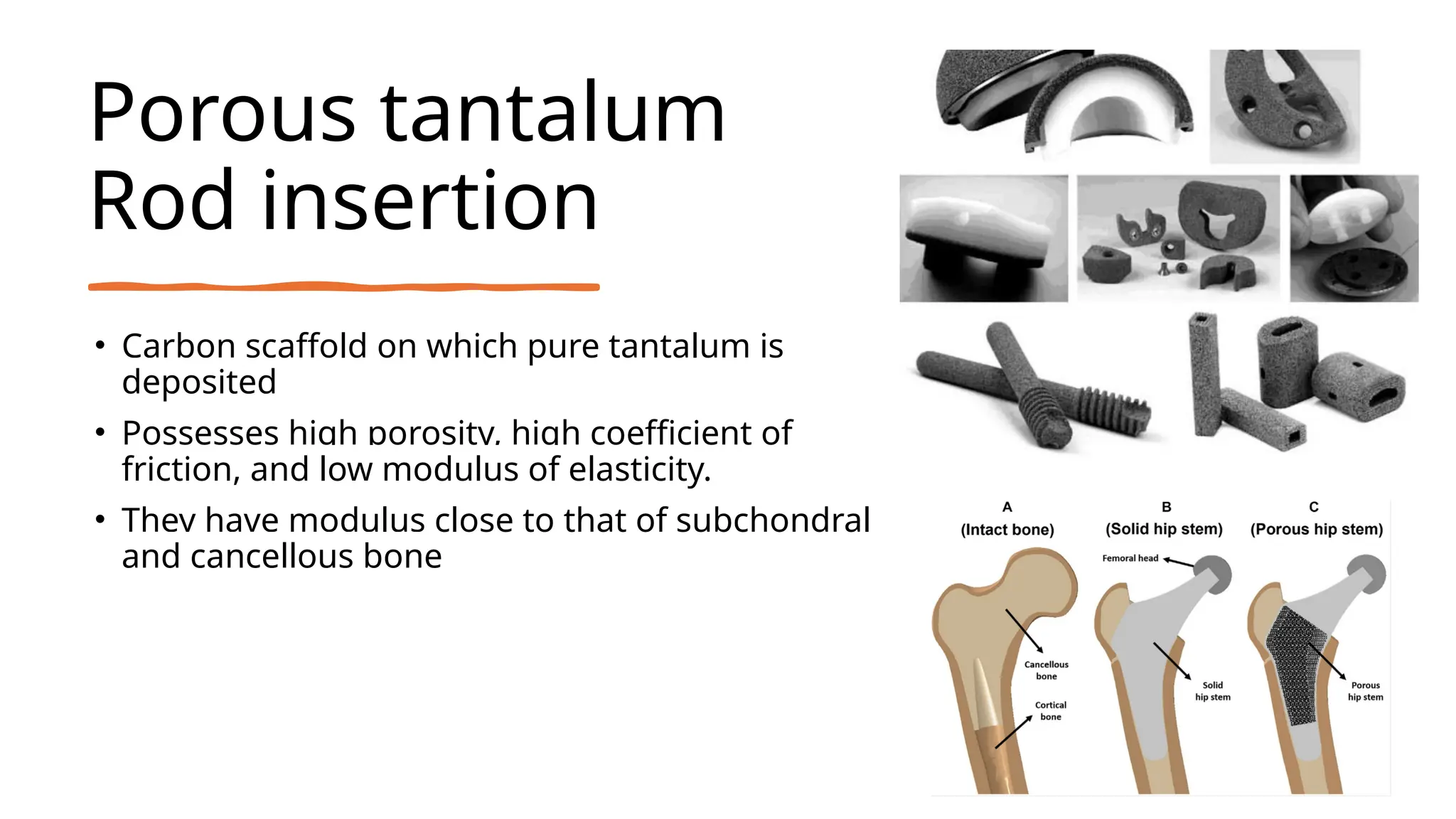
The document discusses platelet-rich plasma (PRP) as an autologous healing method utilizing growth factors, alongside the preparation, use, and complications associated with PRP in orthopaedics. It also explores bio-inert biomaterials used in orthopaedic implants, their classifications, properties, and applications, as well as recent advancements in these materials. Key topics include the efficacy of PRP, diverse biomaterial types, and their biological interactions and challenges.