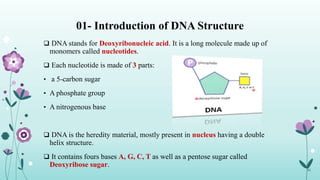
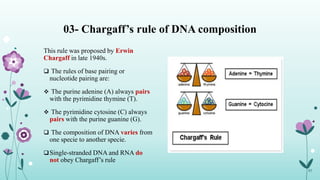
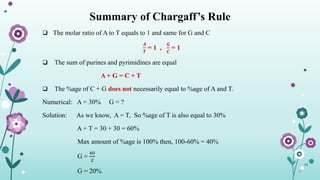
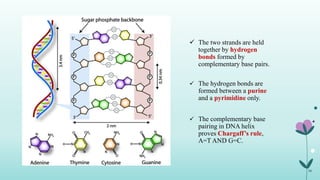
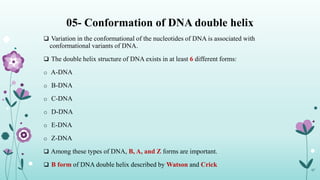
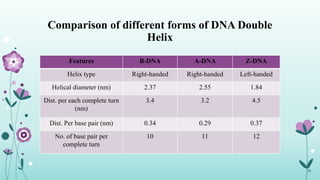
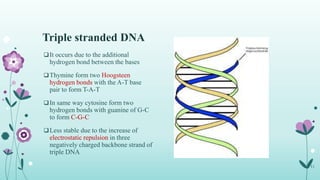
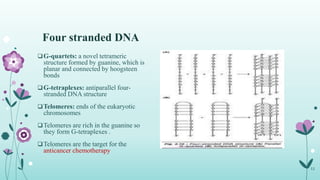
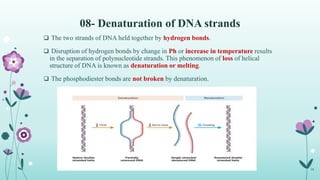
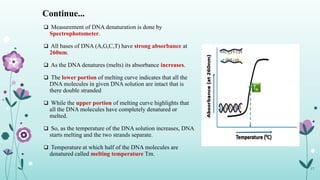
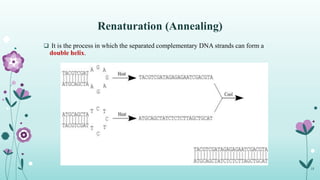
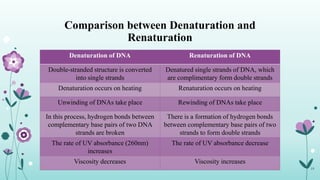
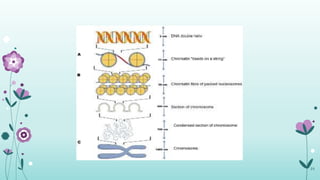
The document provides an extensive overview of deoxyribonucleic acid (DNA), covering its structure, composition, and various forms such as the double helix model proposed by Watson and Crick. It discusses Chargaff's rule of DNA composition, DNA denaturation and renaturation, as well as the organization of DNA in prokaryotic and eukaryotic cells. Key features like the types of DNA structures and the physical properties and functions of DNA are also highlighted.