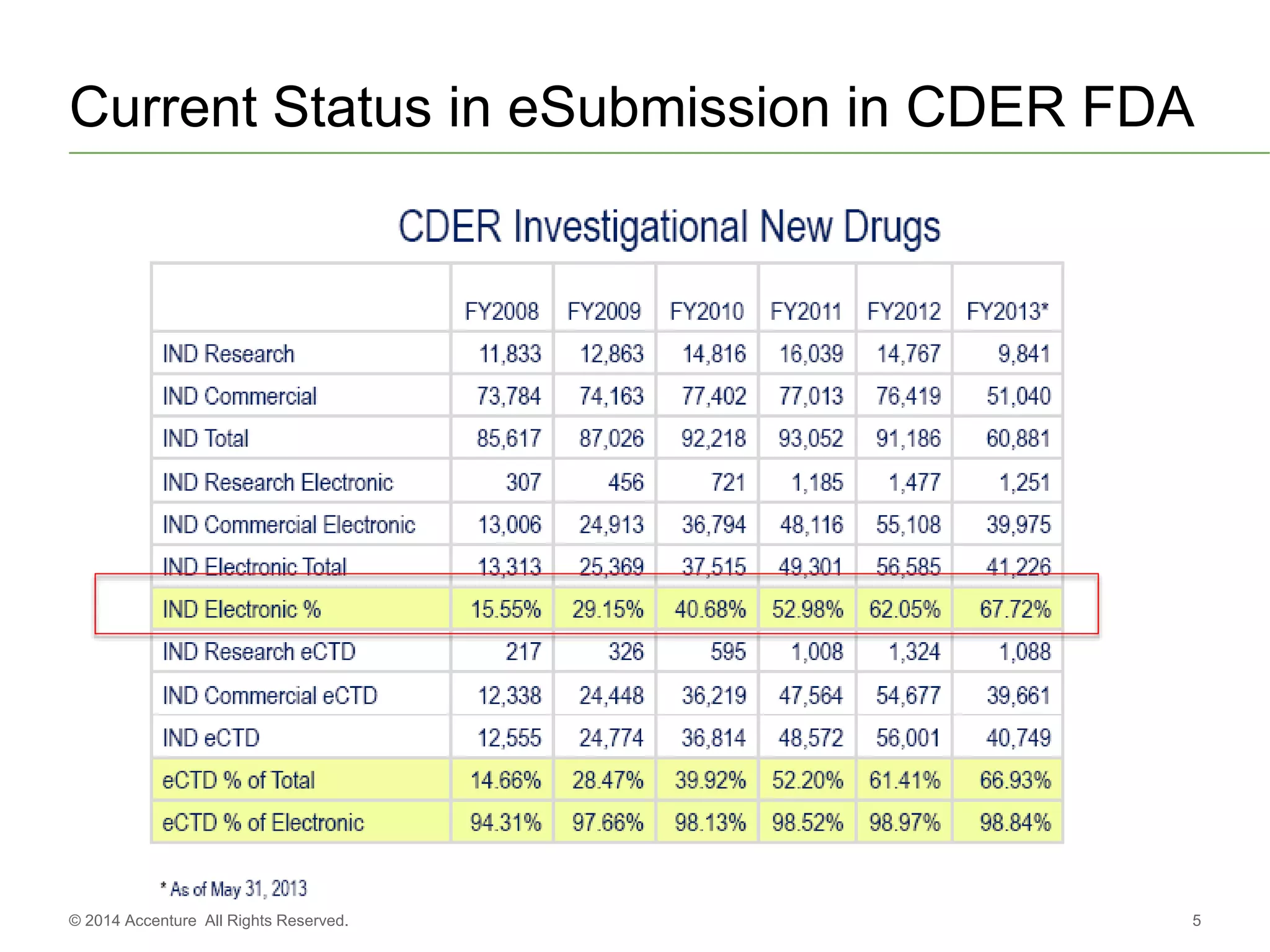
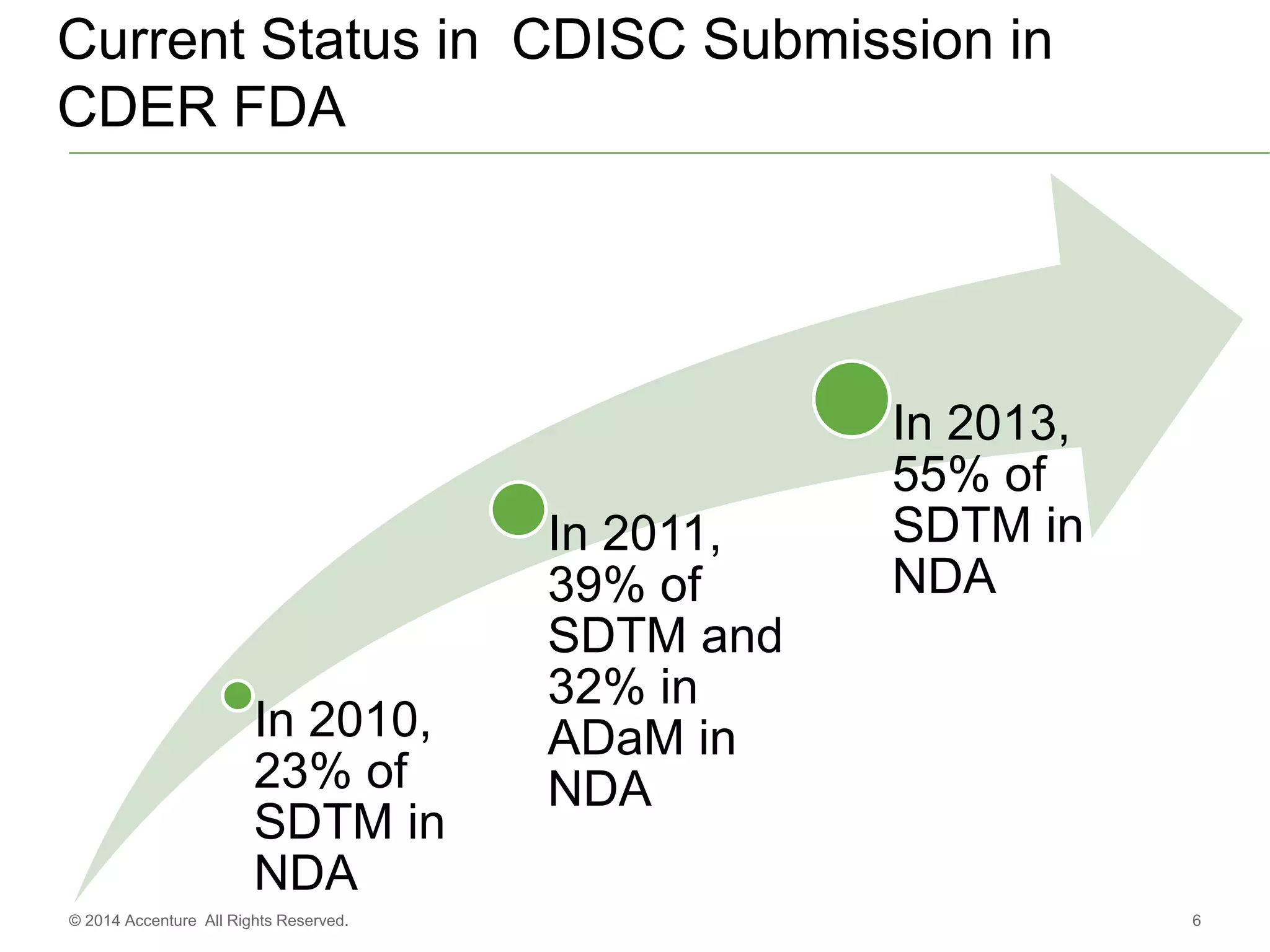
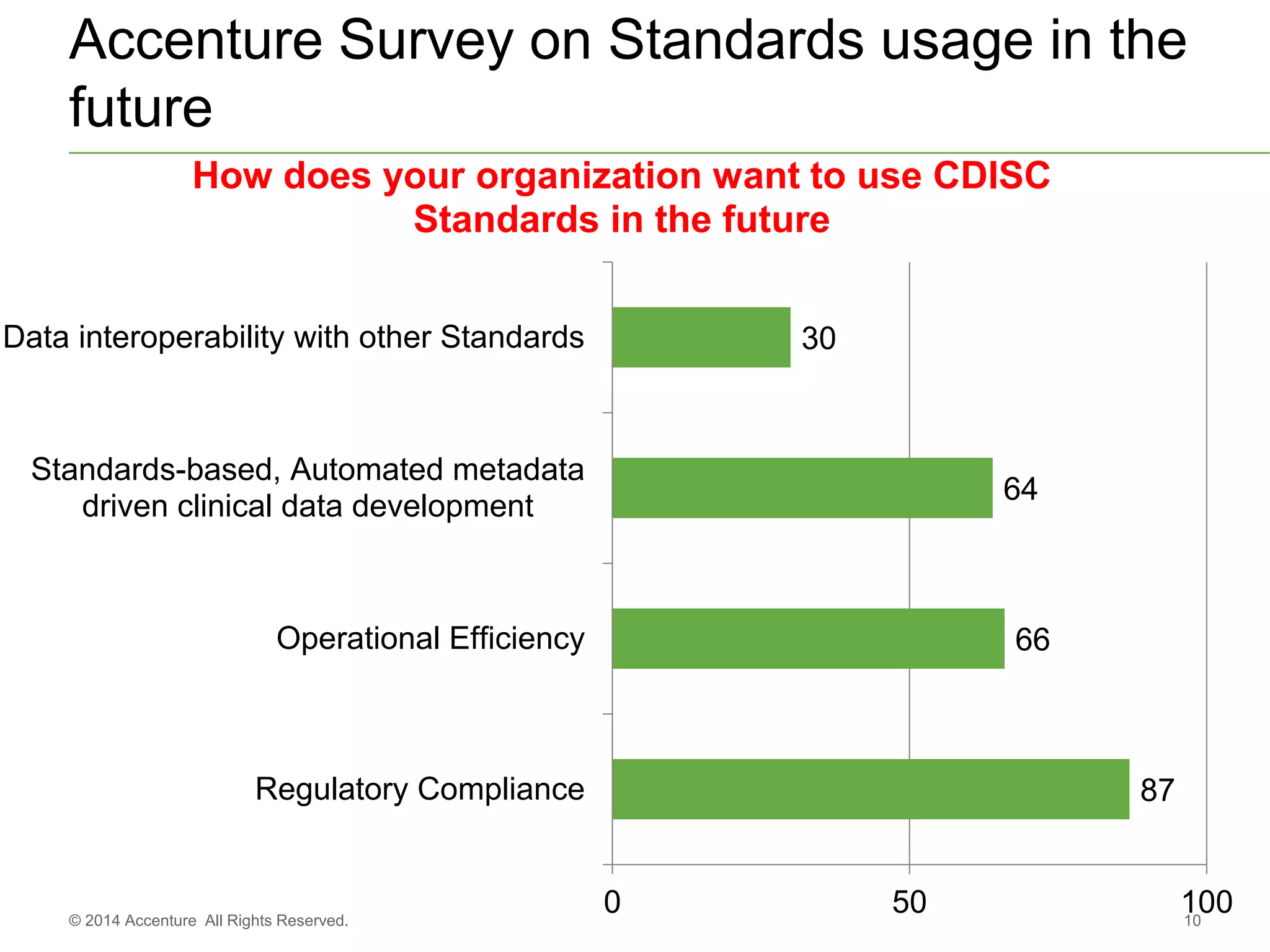
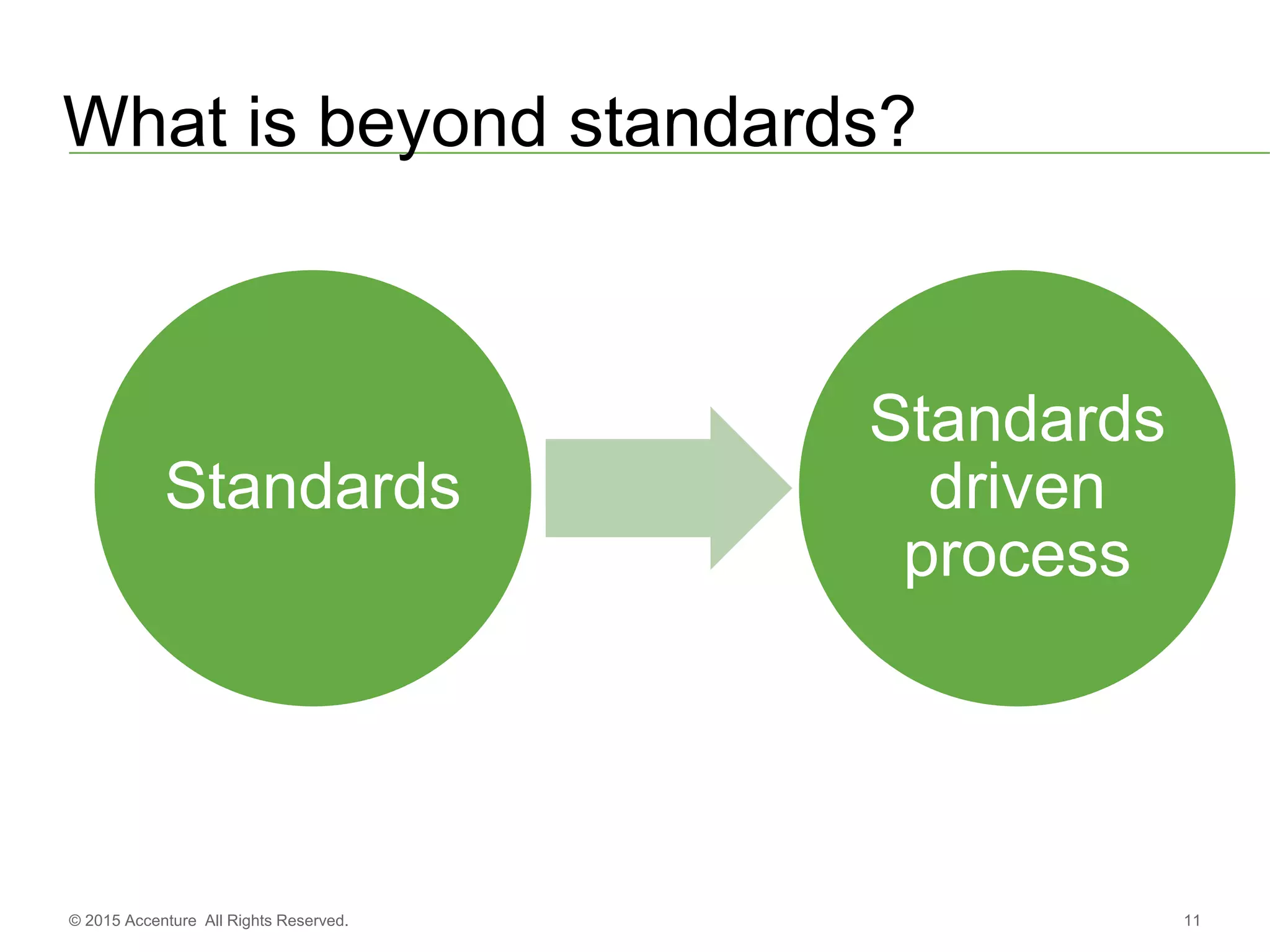
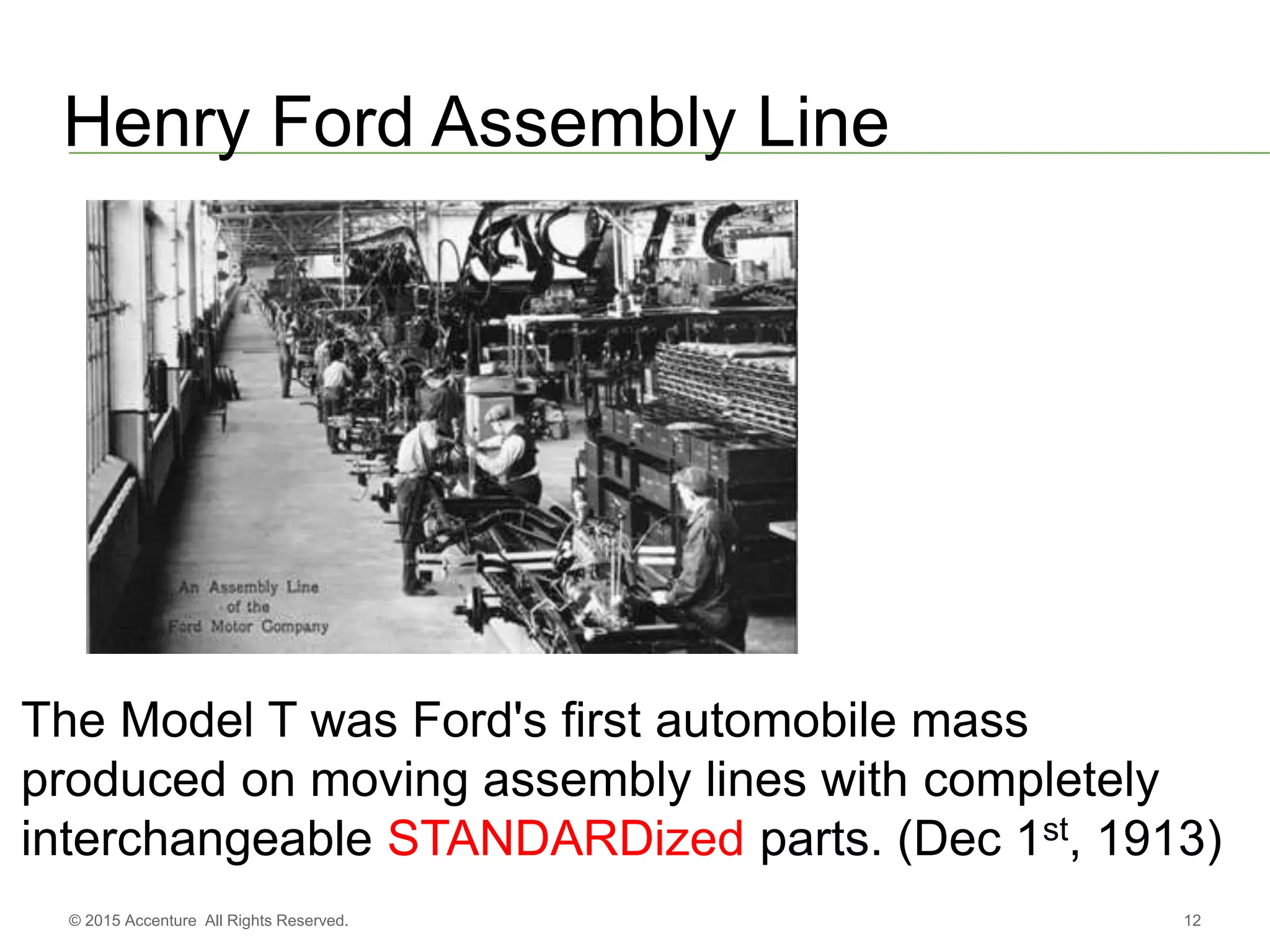
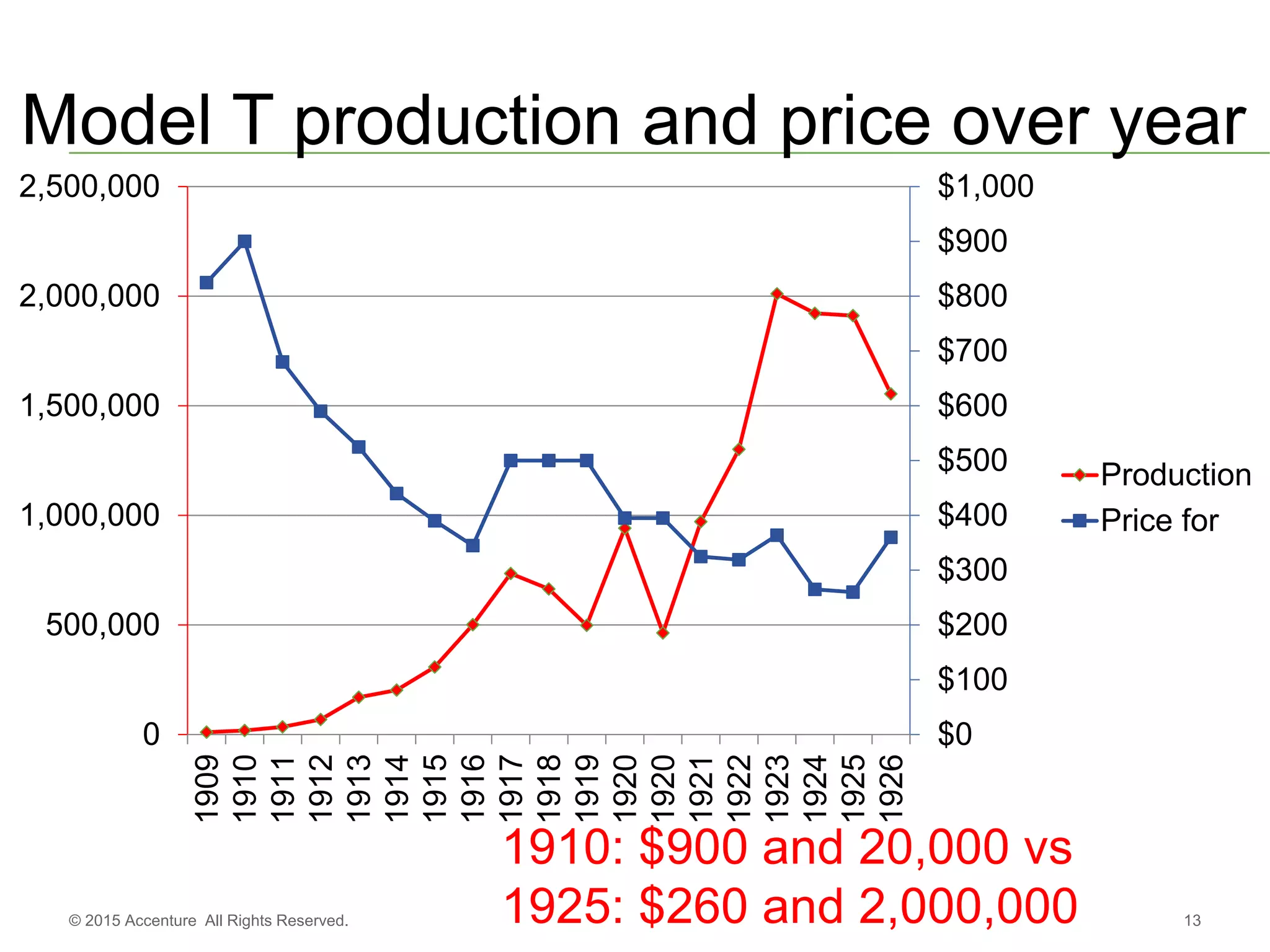
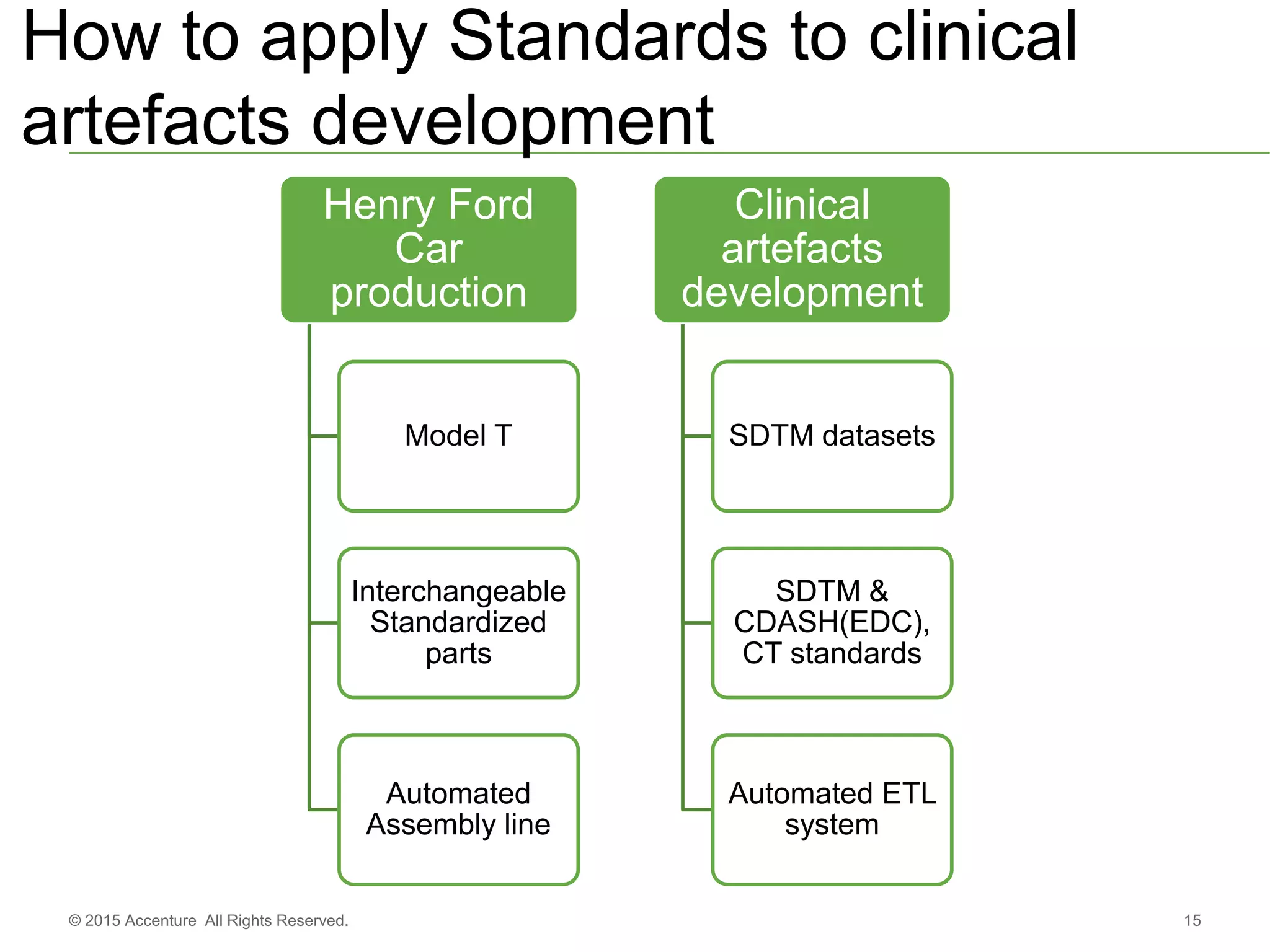
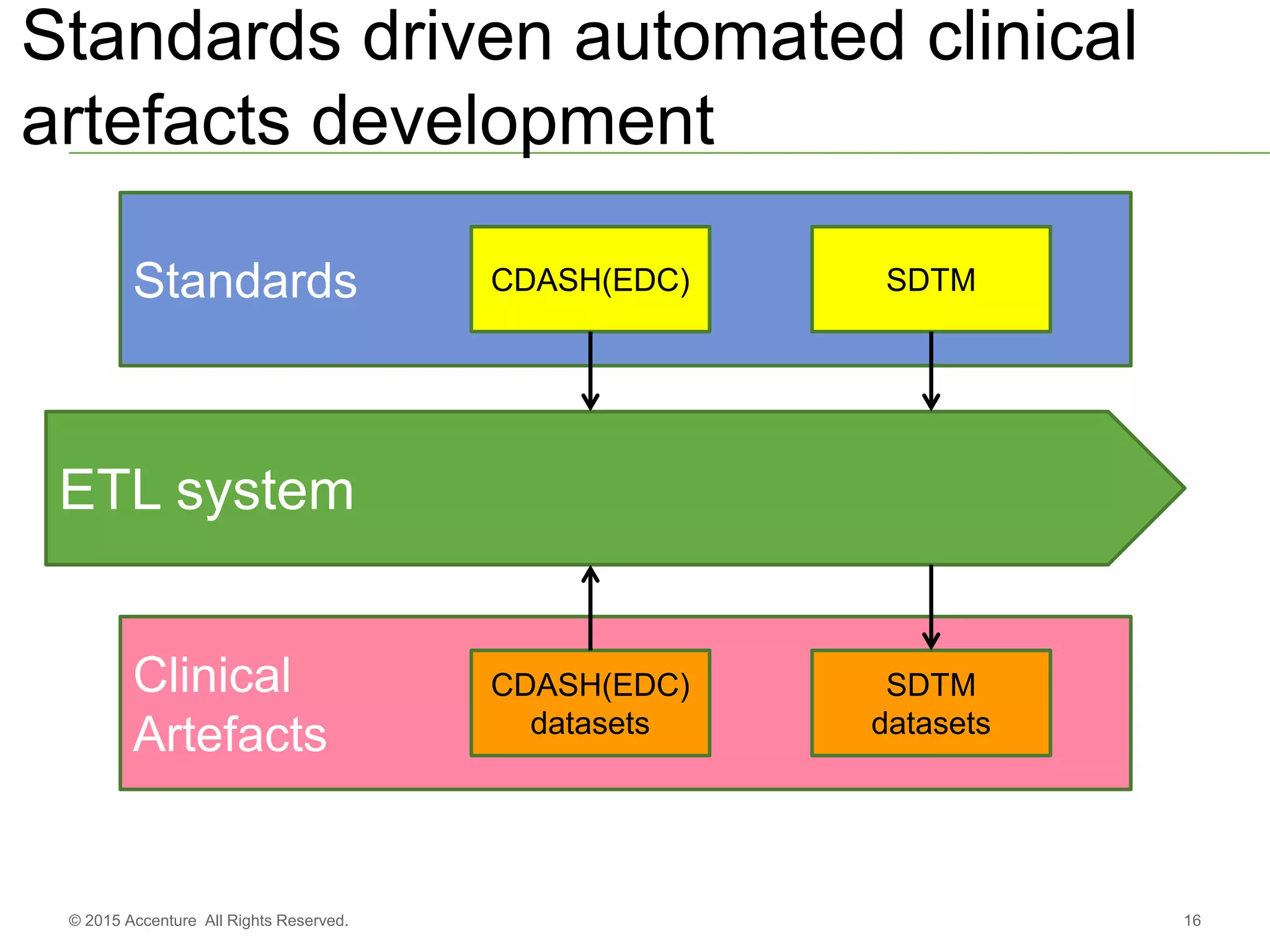
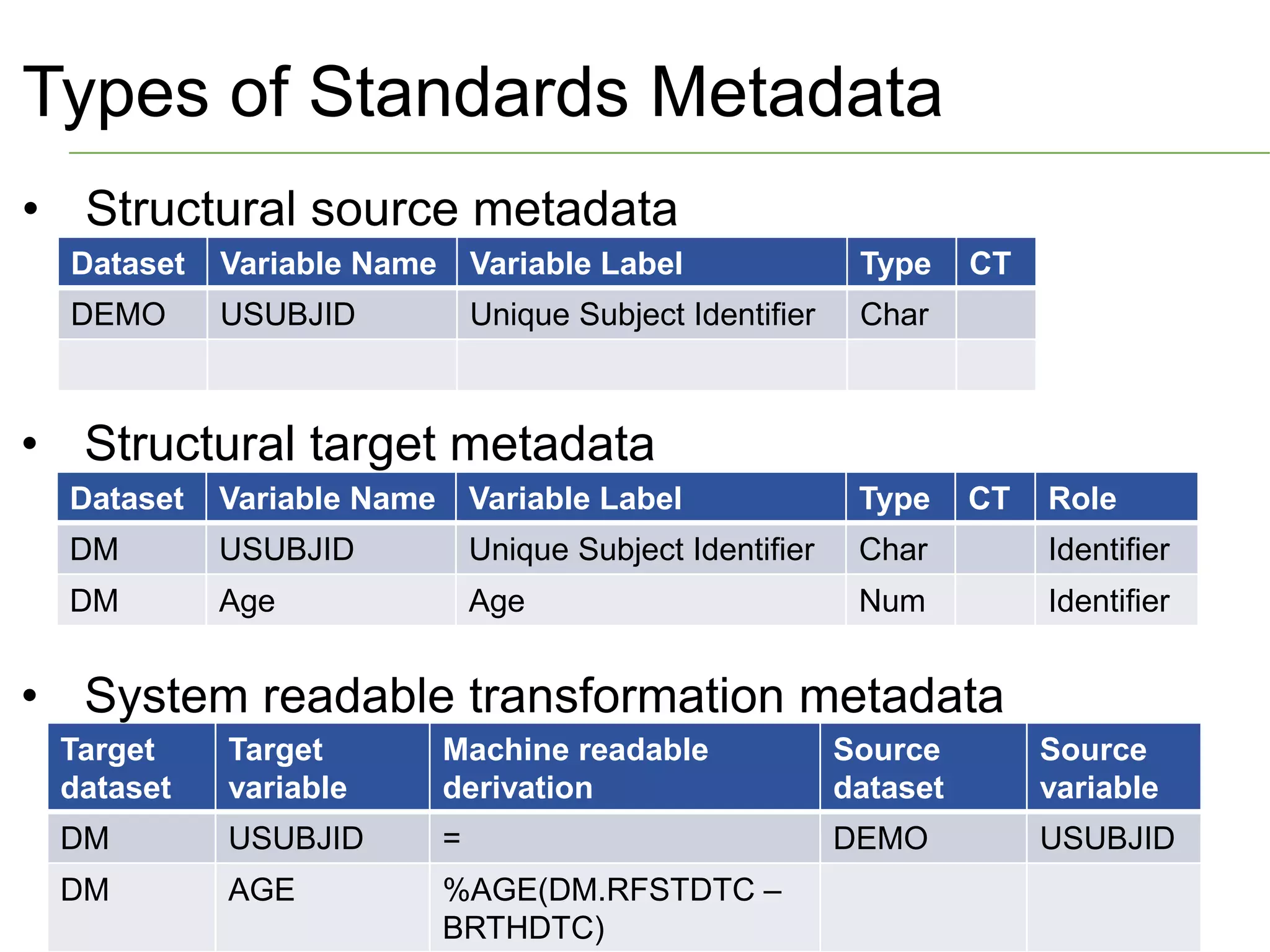
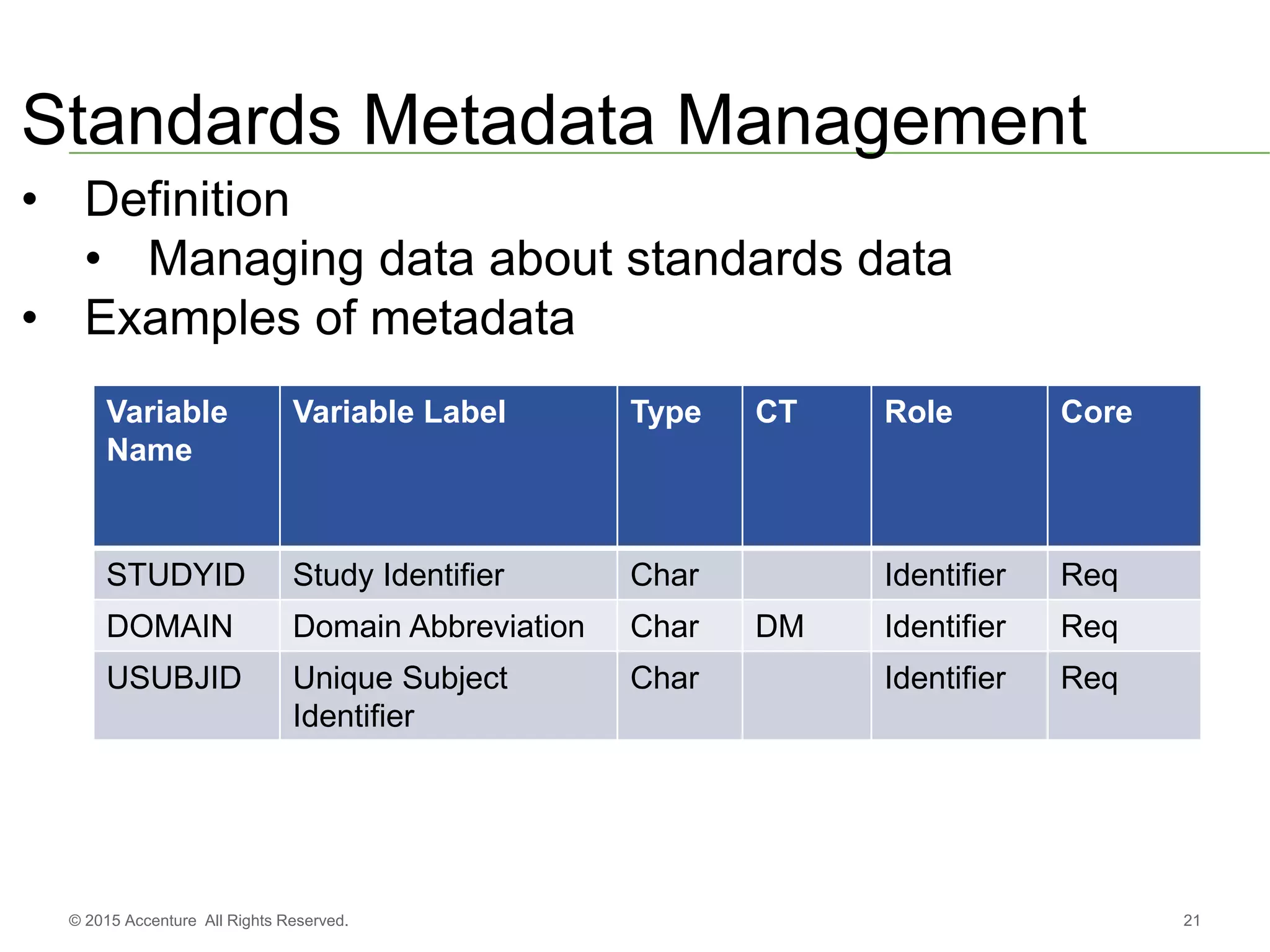
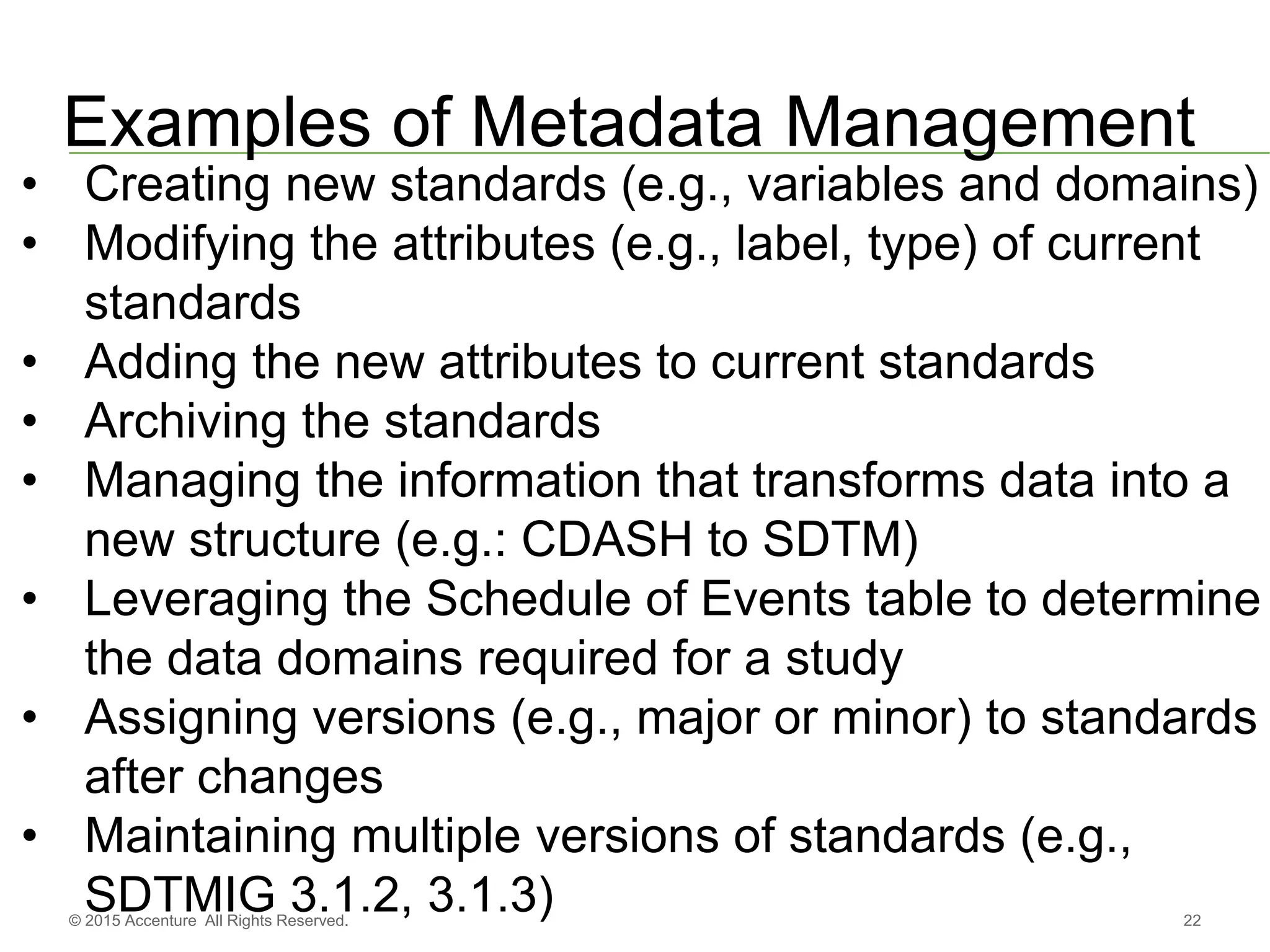
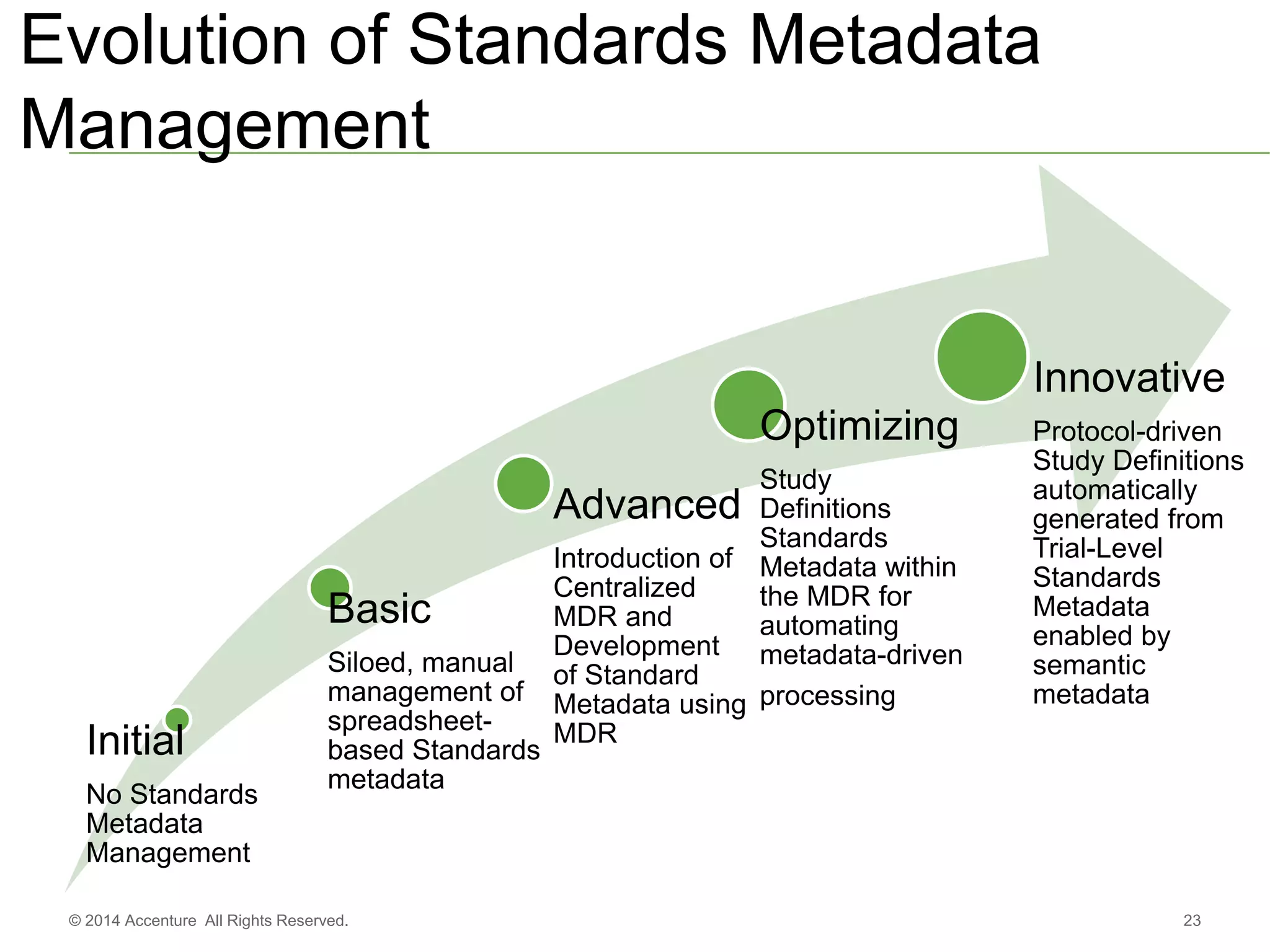
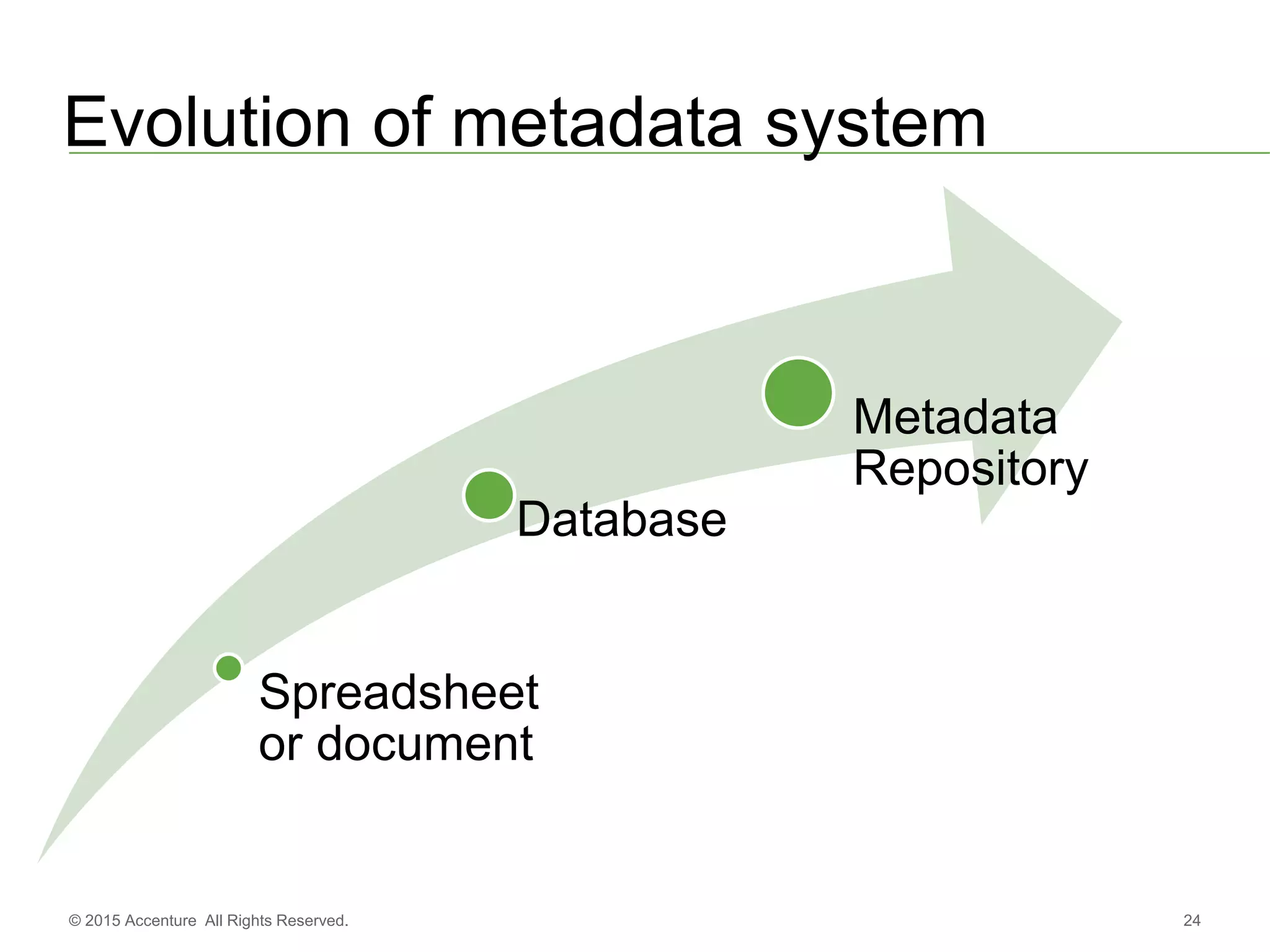
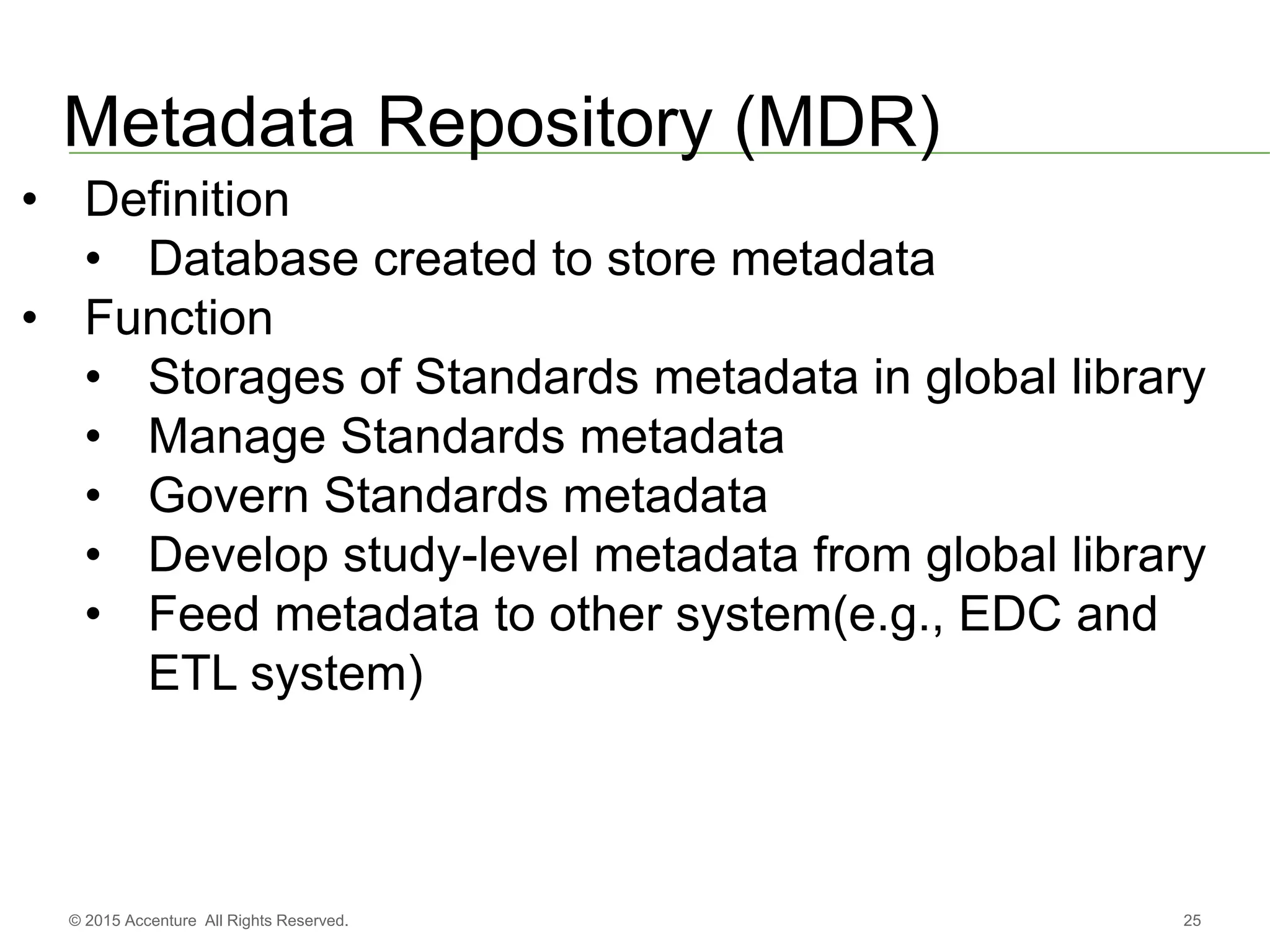
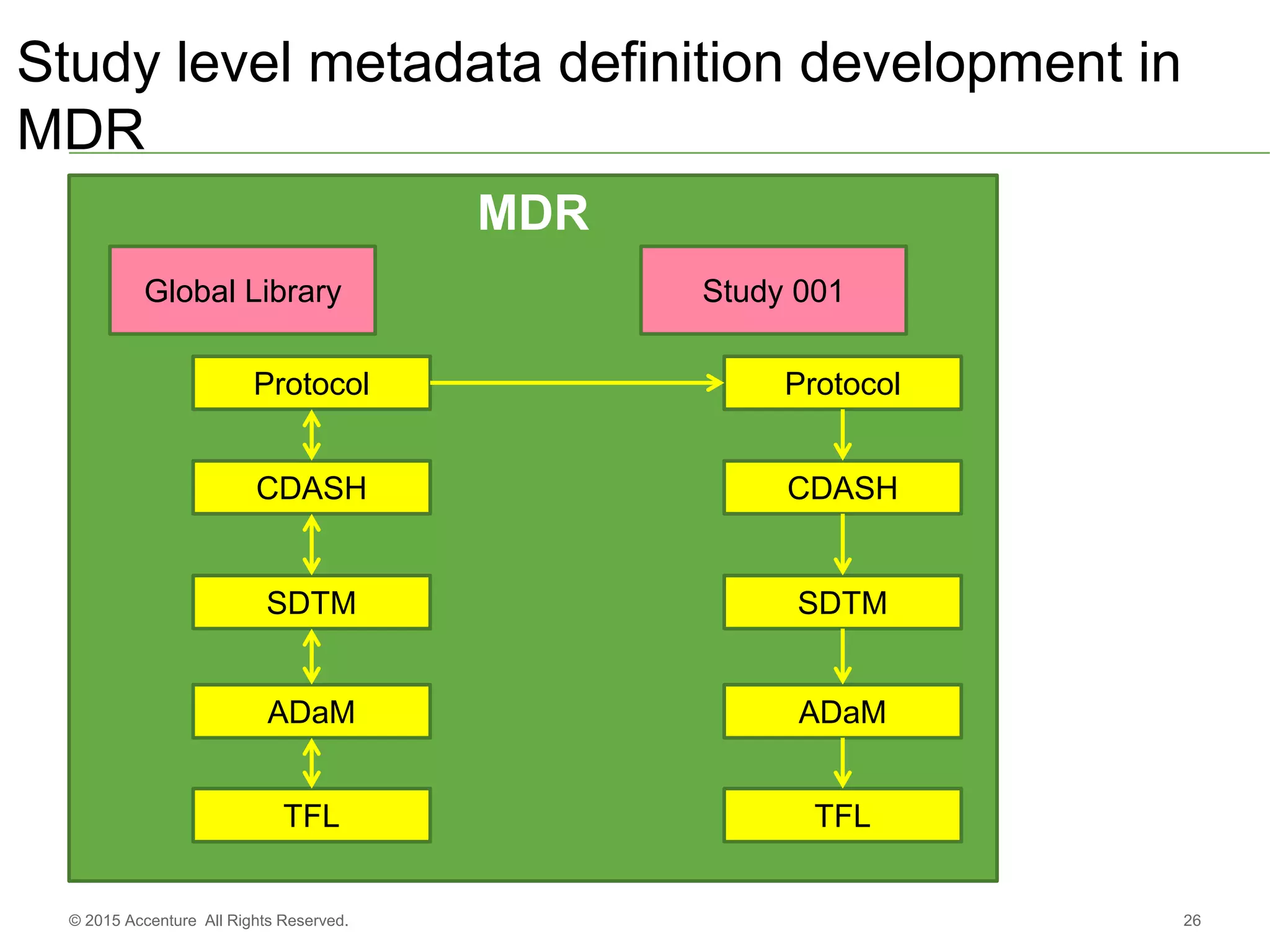
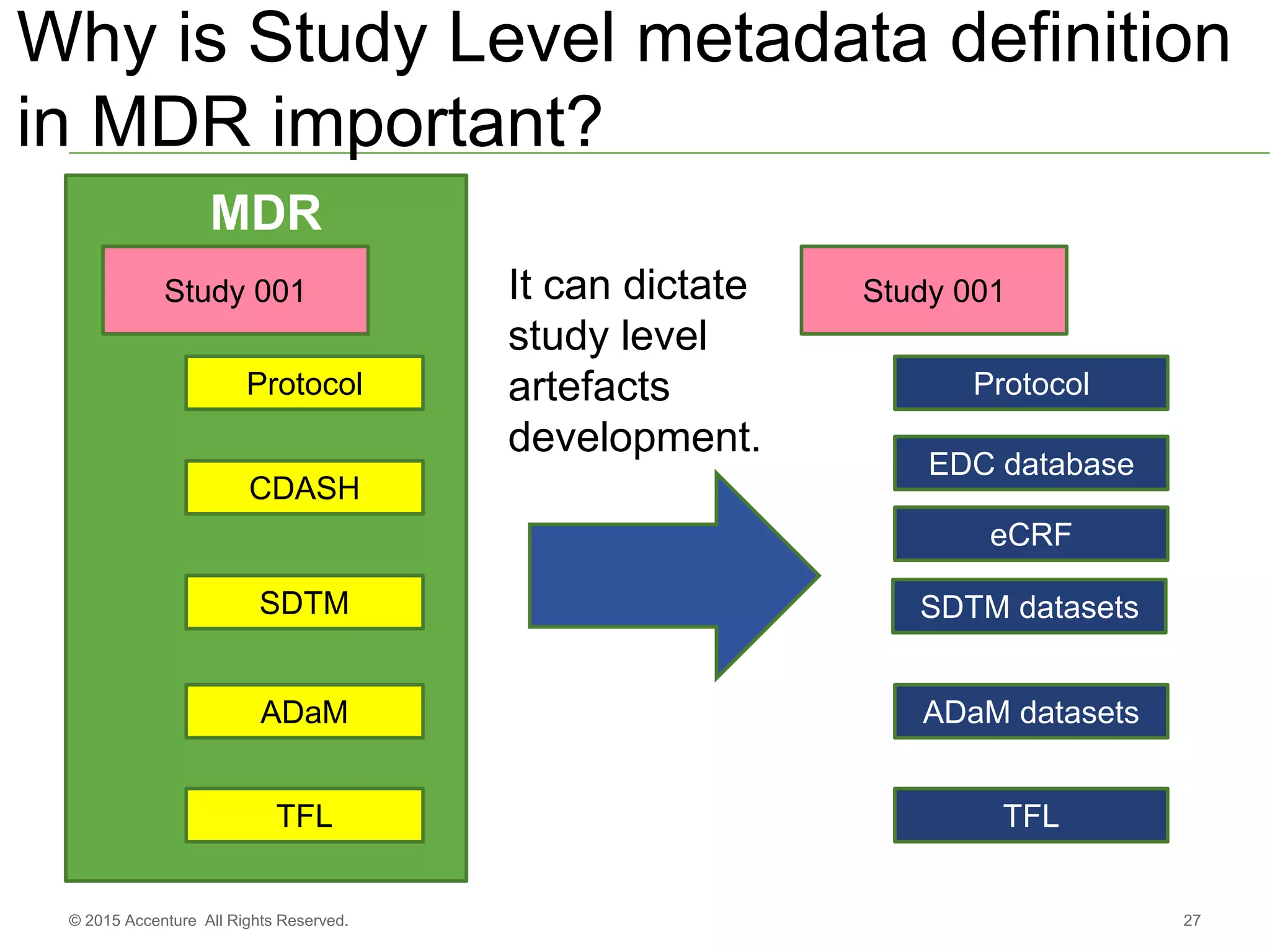
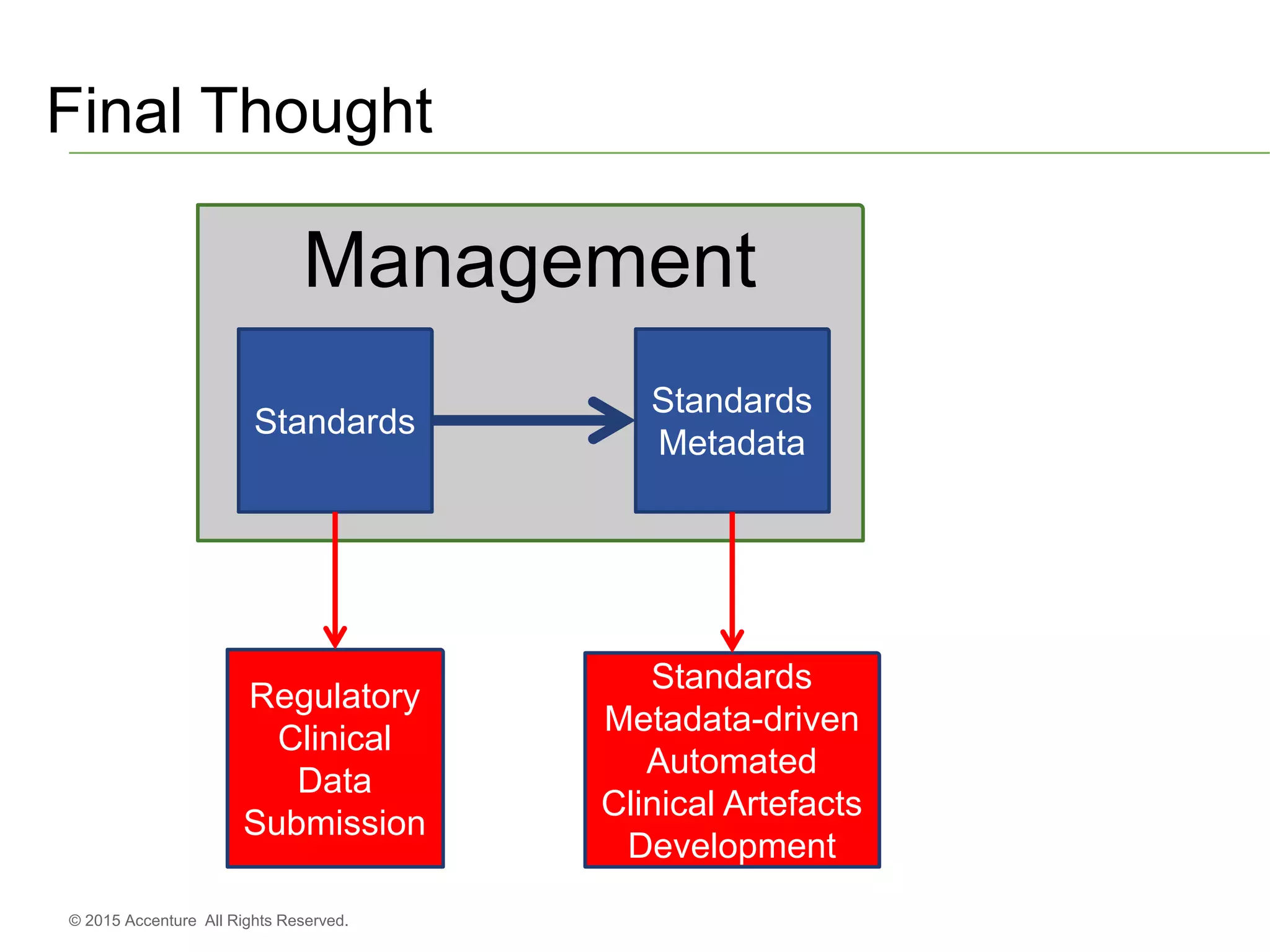
The document discusses standards metadata management and regulatory requirements for clinical data submission, specifically focusing on CDISC standards. It highlights the evolution of standards metadata management, including the introduction of a centralized metadata repository (MDR) and the need for automated clinical artifacts development processes. Additionally, it emphasizes the importance of managing and evolving standards metadata to enhance operational efficiency and improve regulatory compliance in clinical trials.