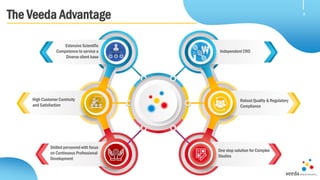
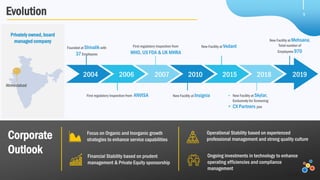
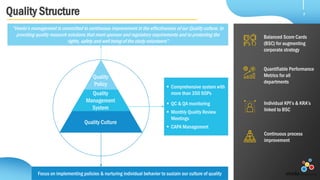
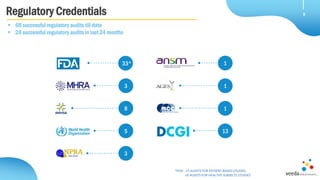
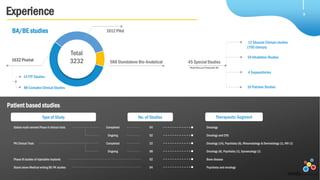
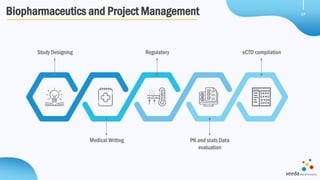
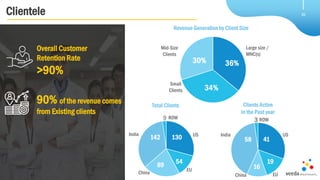
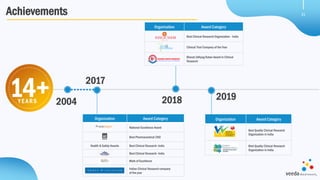
Veeda is a global clinical research organization focusing on quality and regulatory compliance, supporting a diverse client base with extensive scientific competence. The company is dedicated to continuous improvement, evidenced by its successful regulatory audits and a strong emphasis on quality culture and operational stability. Veeda's capabilities cover a wide range of therapeutic areas and complex clinical studies, underpinned by significant investments in infrastructure and technology.