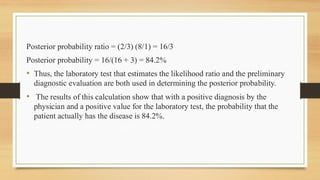
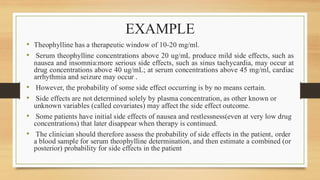
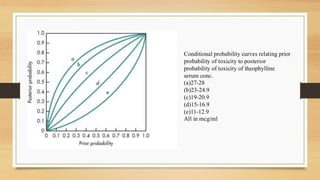
The document discusses Bayesian theory's application in pharmacokinetics for disease diagnosis and drug dosing regimens, emphasizing how prior patient information and new lab data can refine probability assessments. It illustrates this process through examples involving disease probability calculations and theophylline dosing while recognizing Bayesian methods' utility in improving forecasting in medicine. However, it notes the primary disadvantage of subjectivity in selecting prior probabilities, which some statisticians criticize for drug approval purposes.