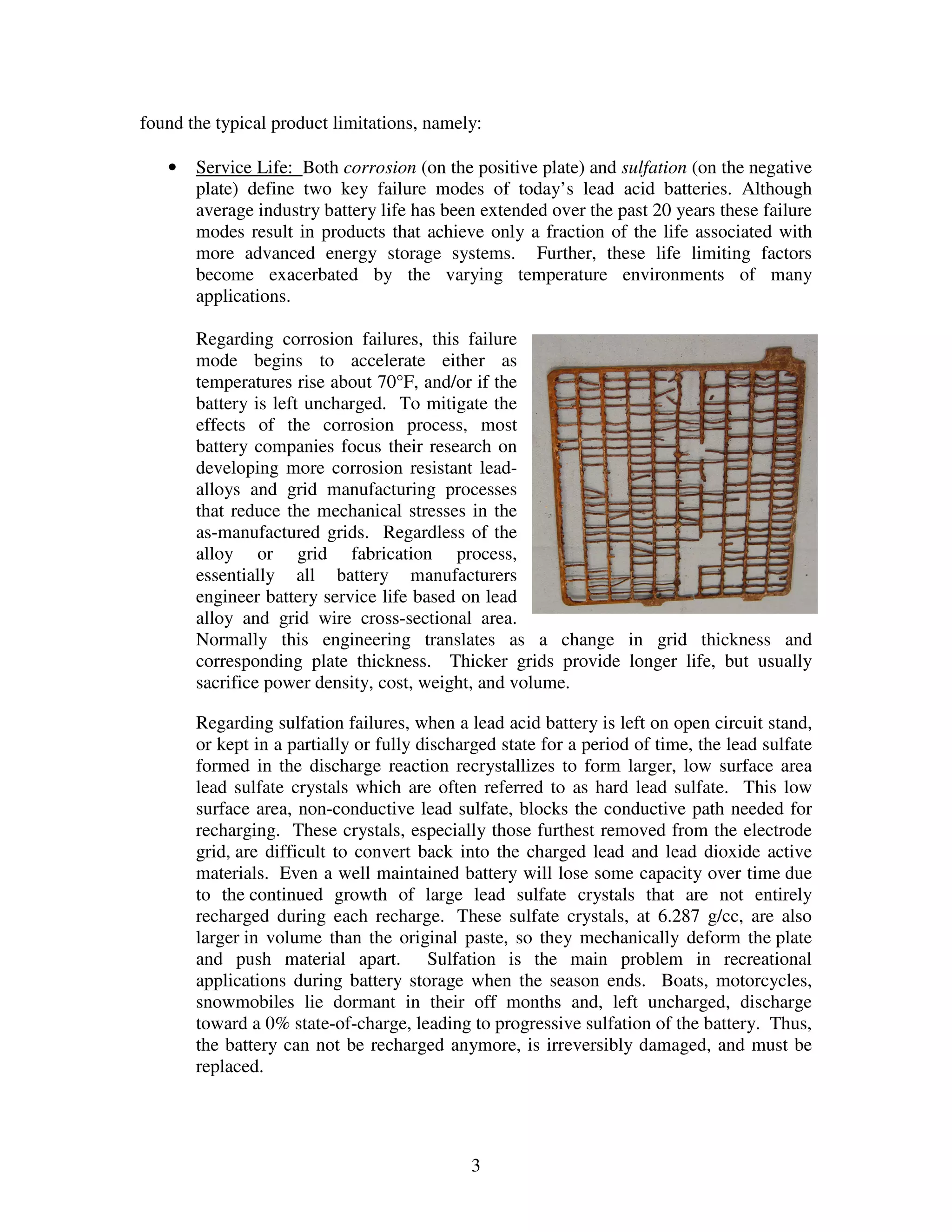
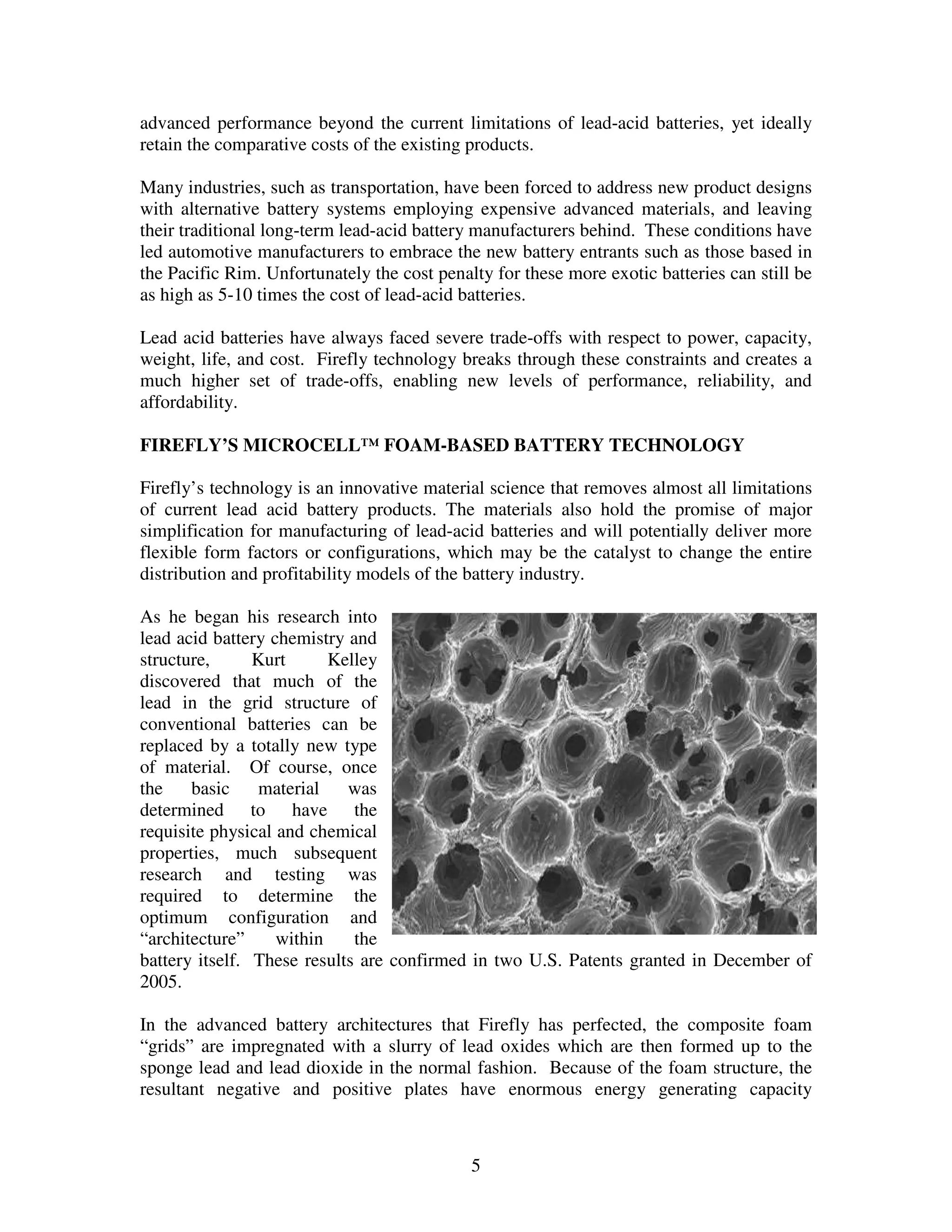
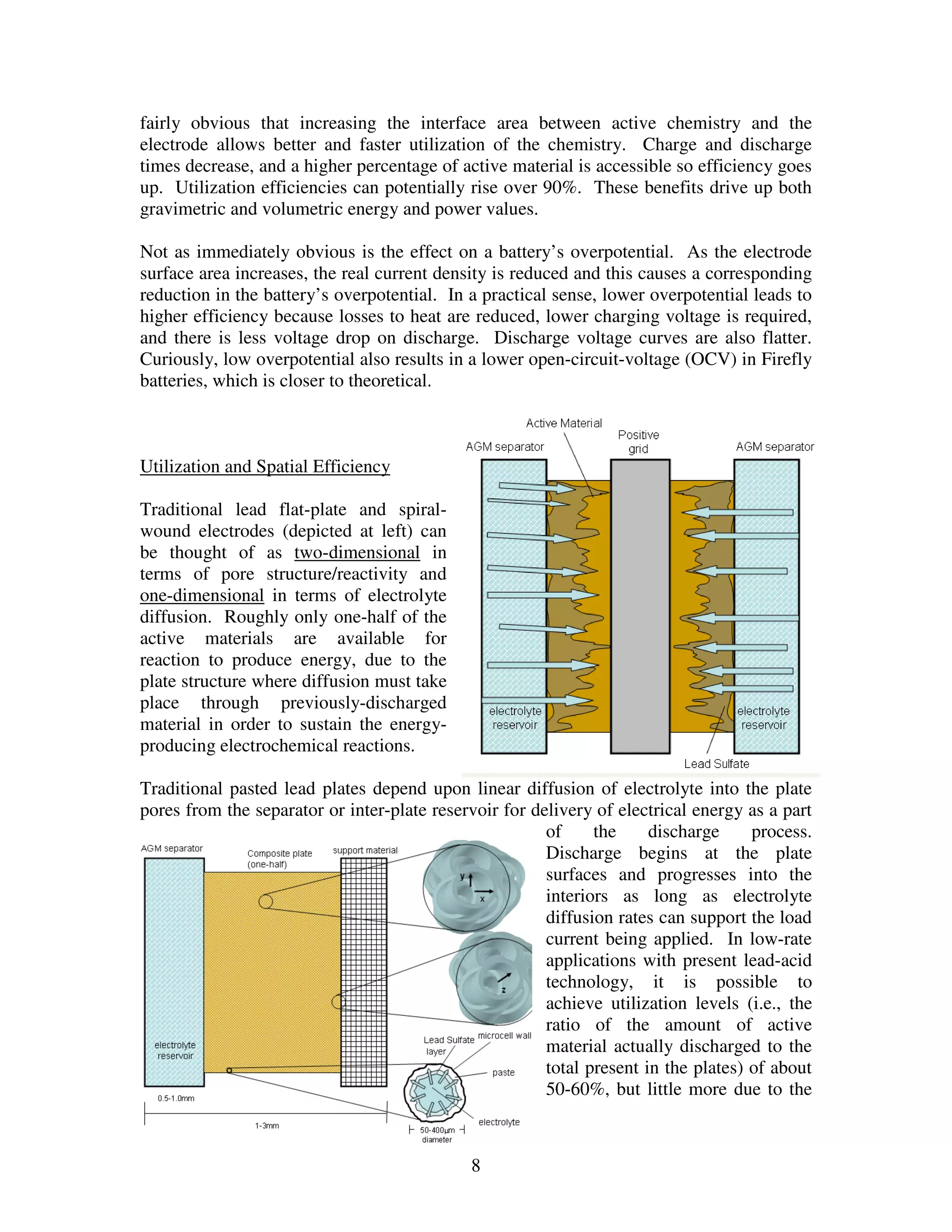
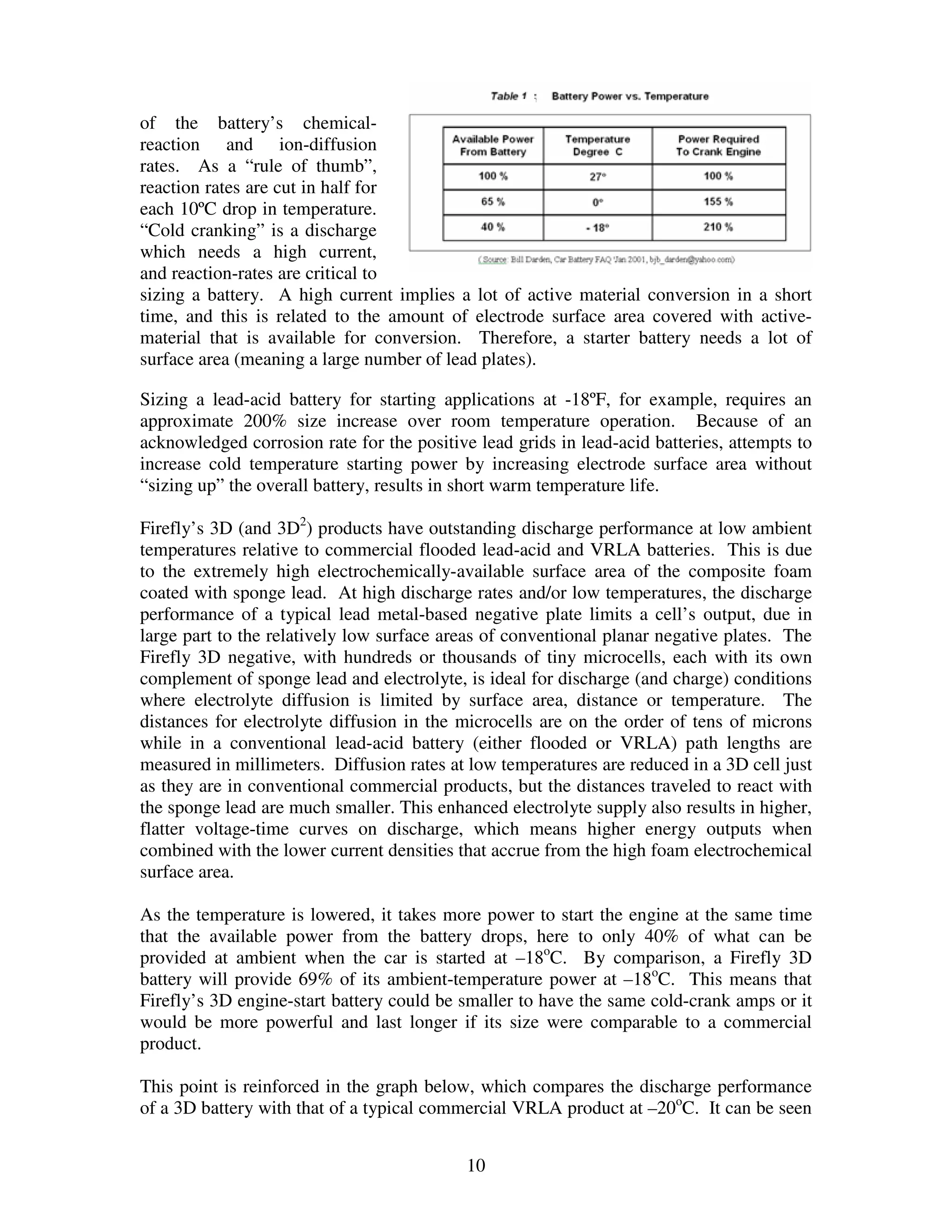
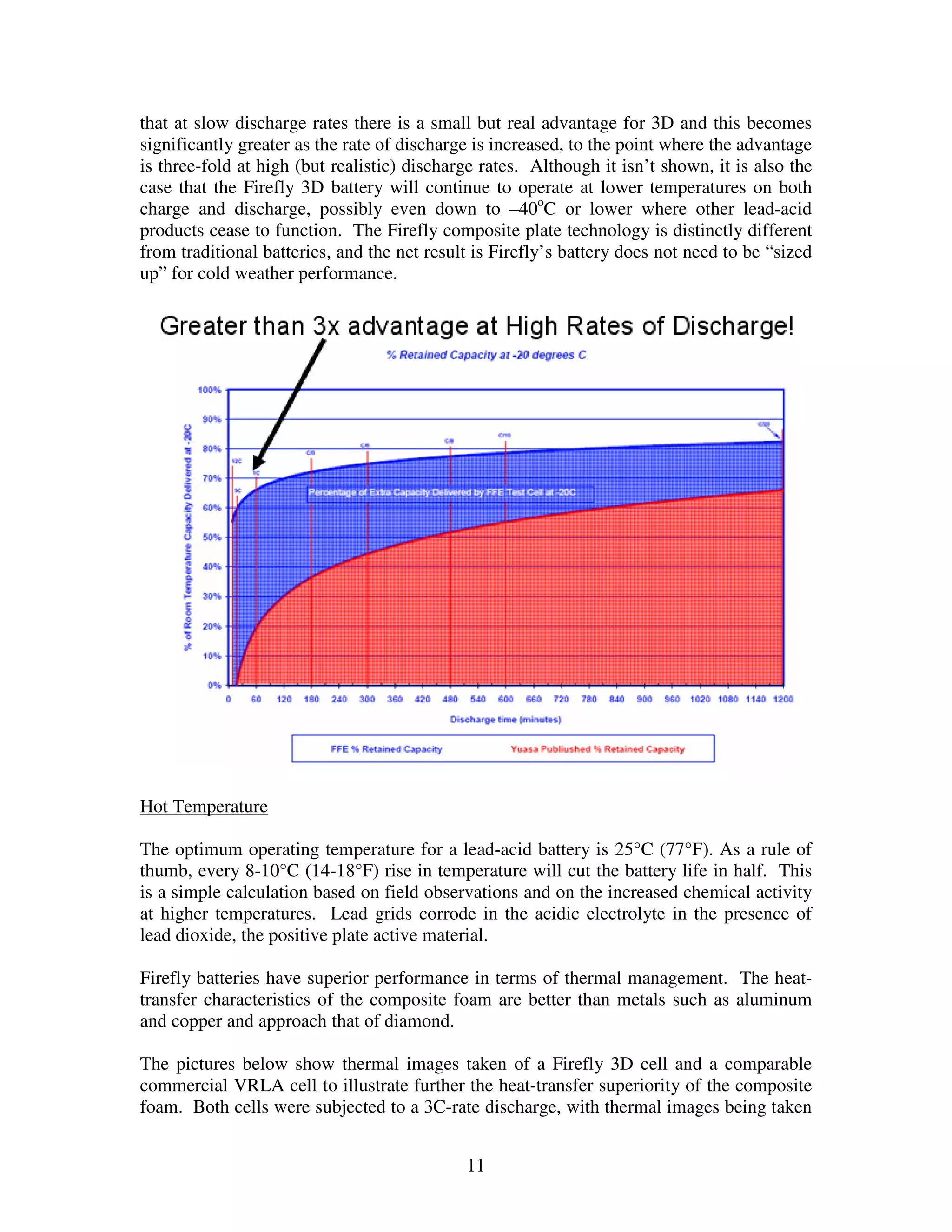
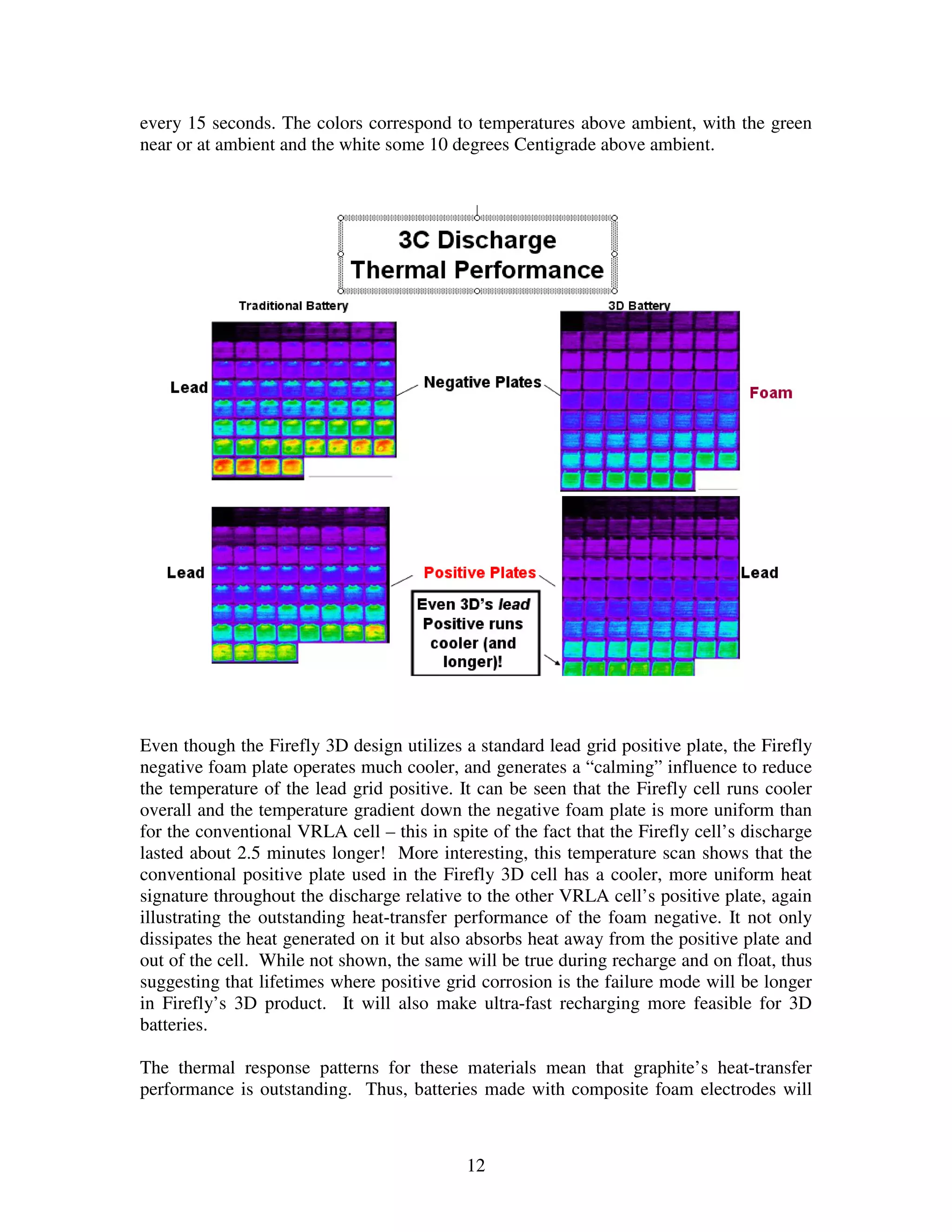
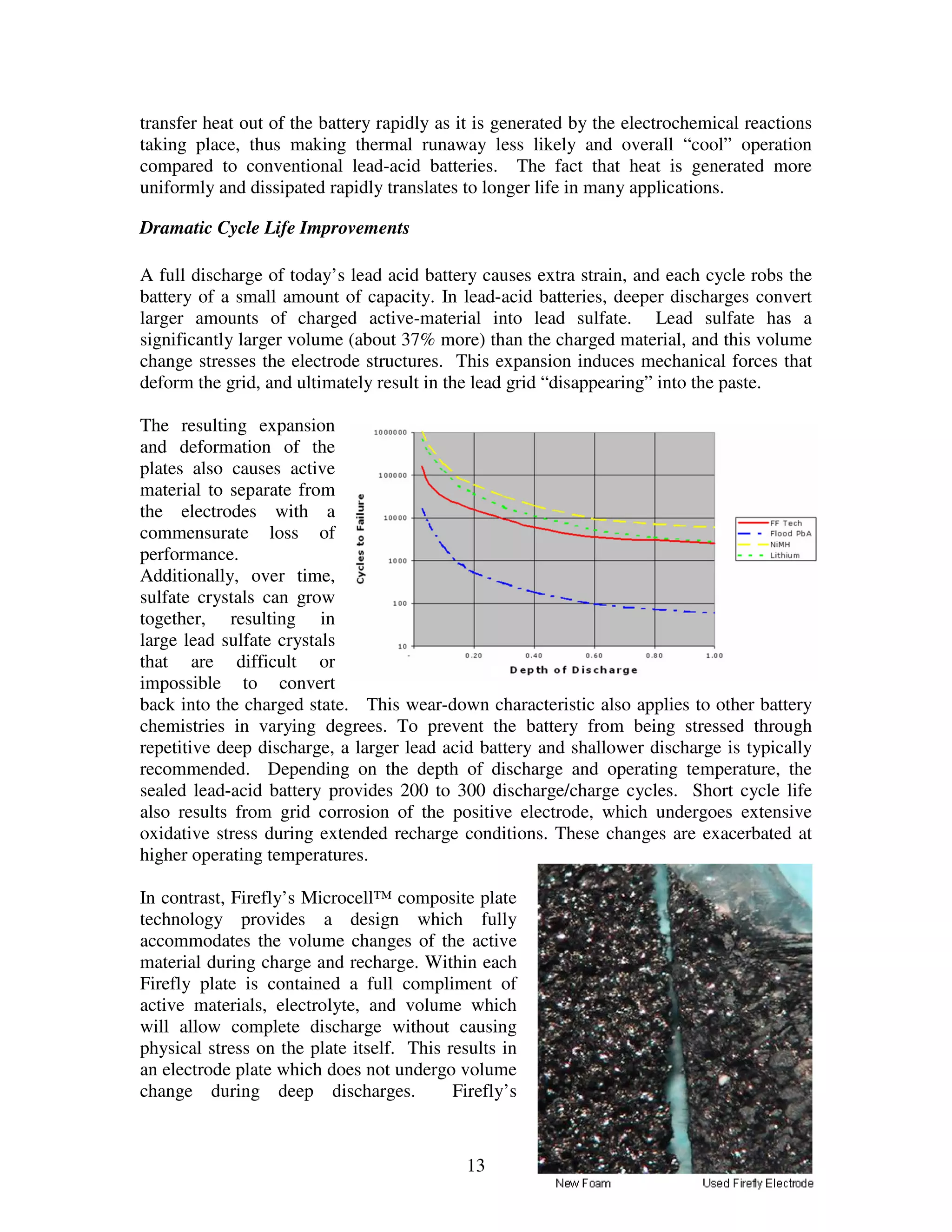
Firefly's revolutionary battery technology originated from research at Caterpillar to improve battery performance for heavy equipment. The technology replaces most of the lead in traditional batteries with a porous foam material called Microcell. This new 3D structure results in batteries with much higher energy density, power, cycle life, and charge rates compared to traditional lead-acid batteries. Firefly batteries can provide similar performance to advanced battery technologies like lithium-ion, but at a much lower cost similar to lead-acid batteries.