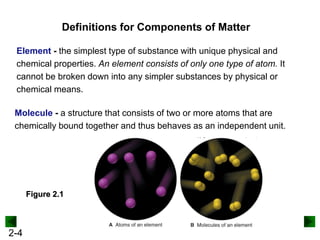
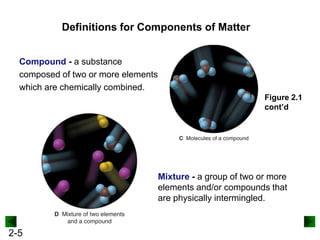
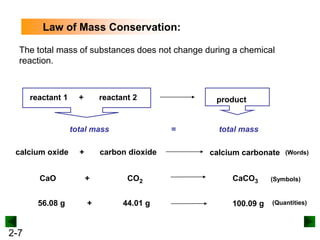
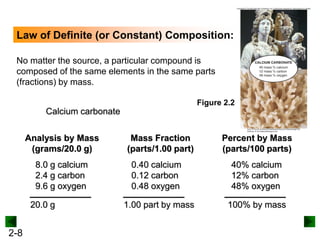
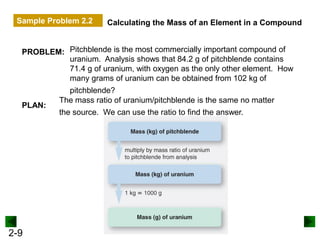
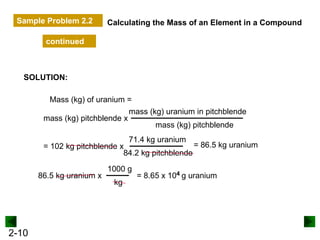
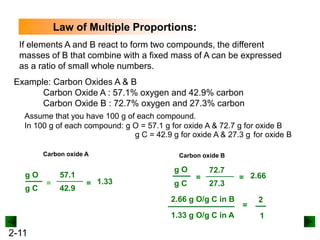
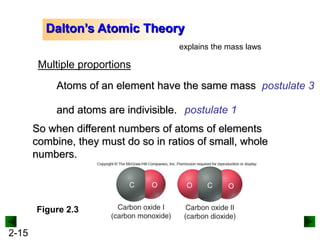
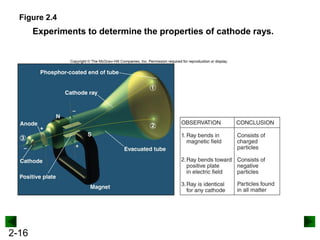
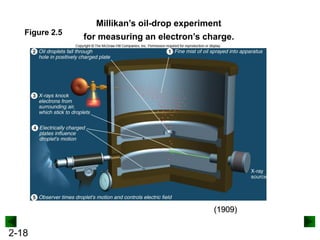
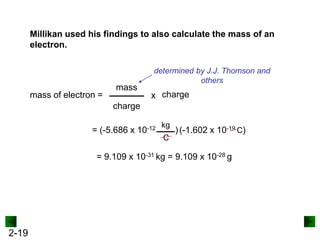
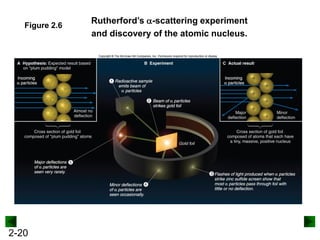
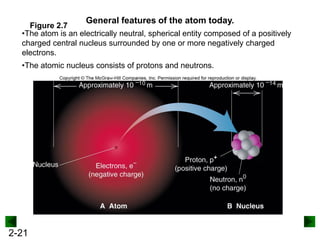
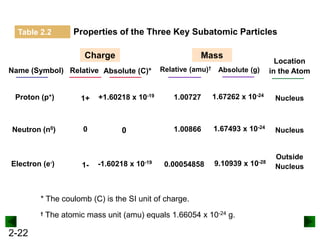
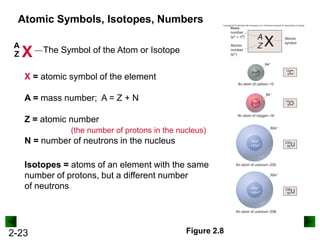
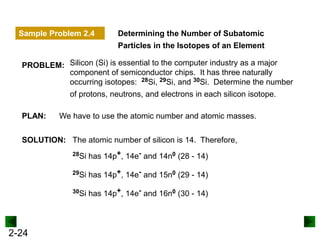
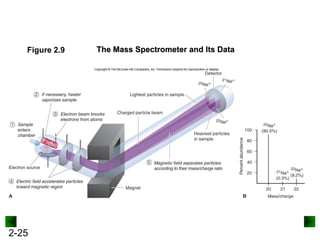
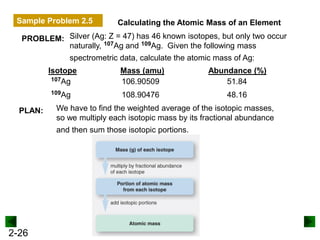
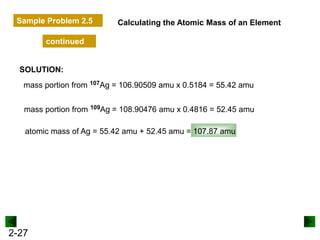
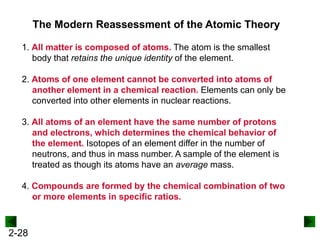
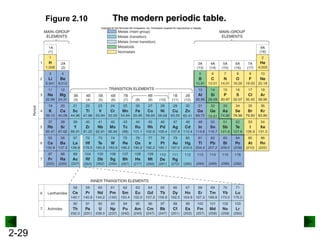
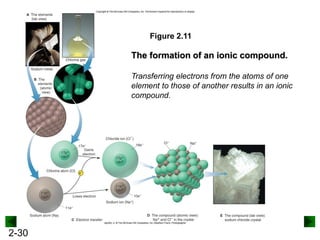
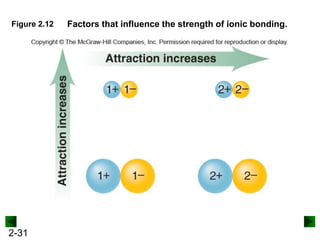
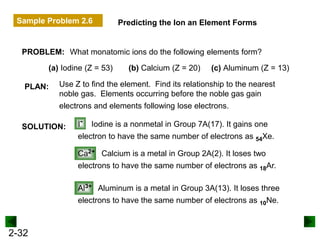
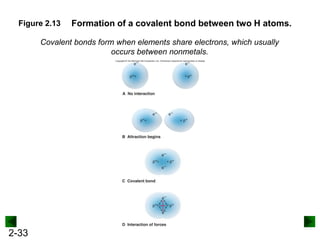
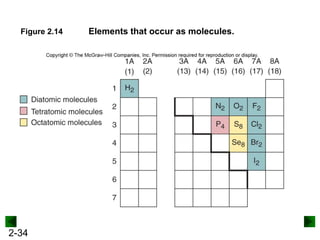
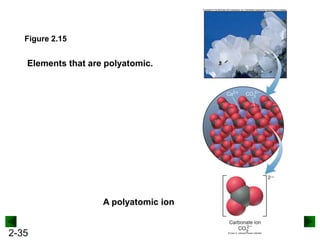
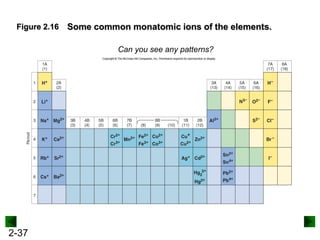
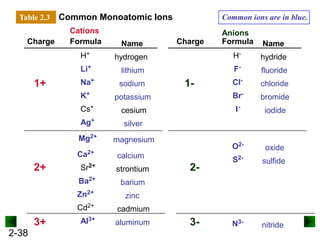
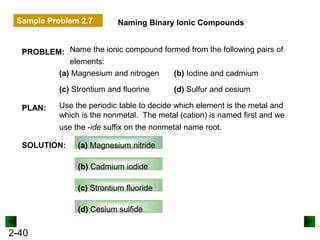
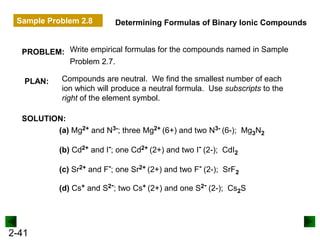
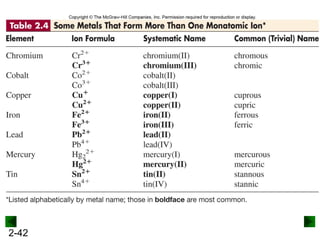
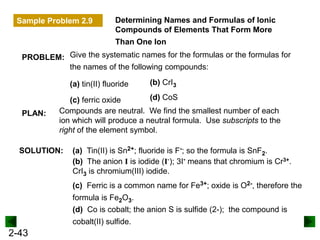
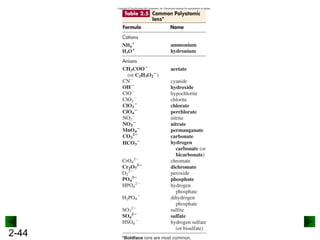
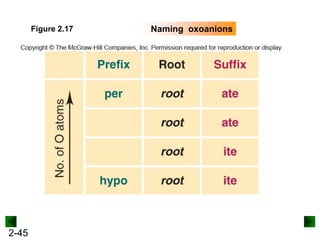
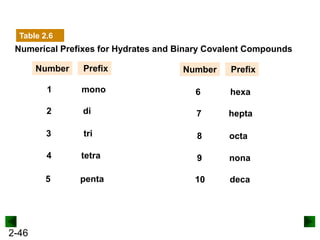
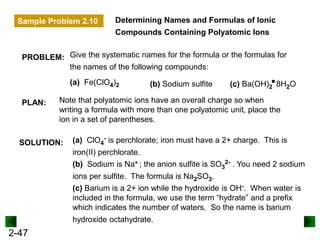
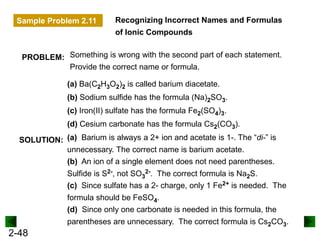
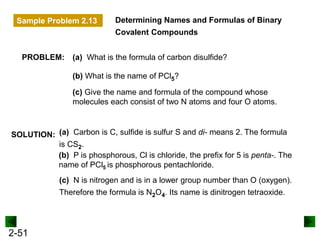
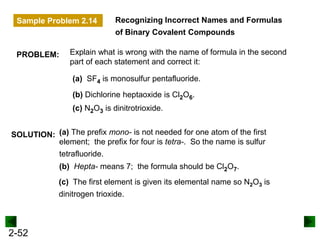
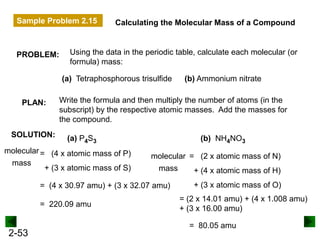
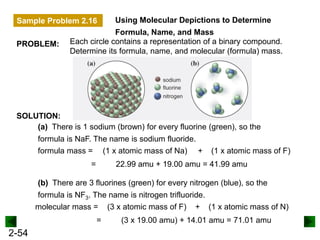
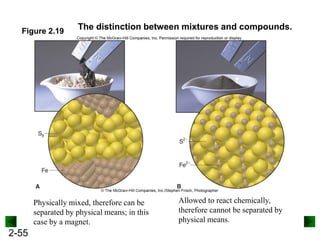
This document provides an overview of advanced inorganic chemistry topics related to the components of matter. It begins with definitions of key terms like element, compound, mixture and discusses Dalton's atomic theory. It then covers historical experiments that helped develop models of the atom, including cathode ray experiments, Millikan's oil drop experiment, and Rutherford's gold foil experiment. The document introduces subatomic particles like protons, neutrons and electrons and explains how they are arranged in atoms. It also discusses isotopes and how atomic mass is calculated. The last sections cover ionic and covalent bonding and how compounds are named.