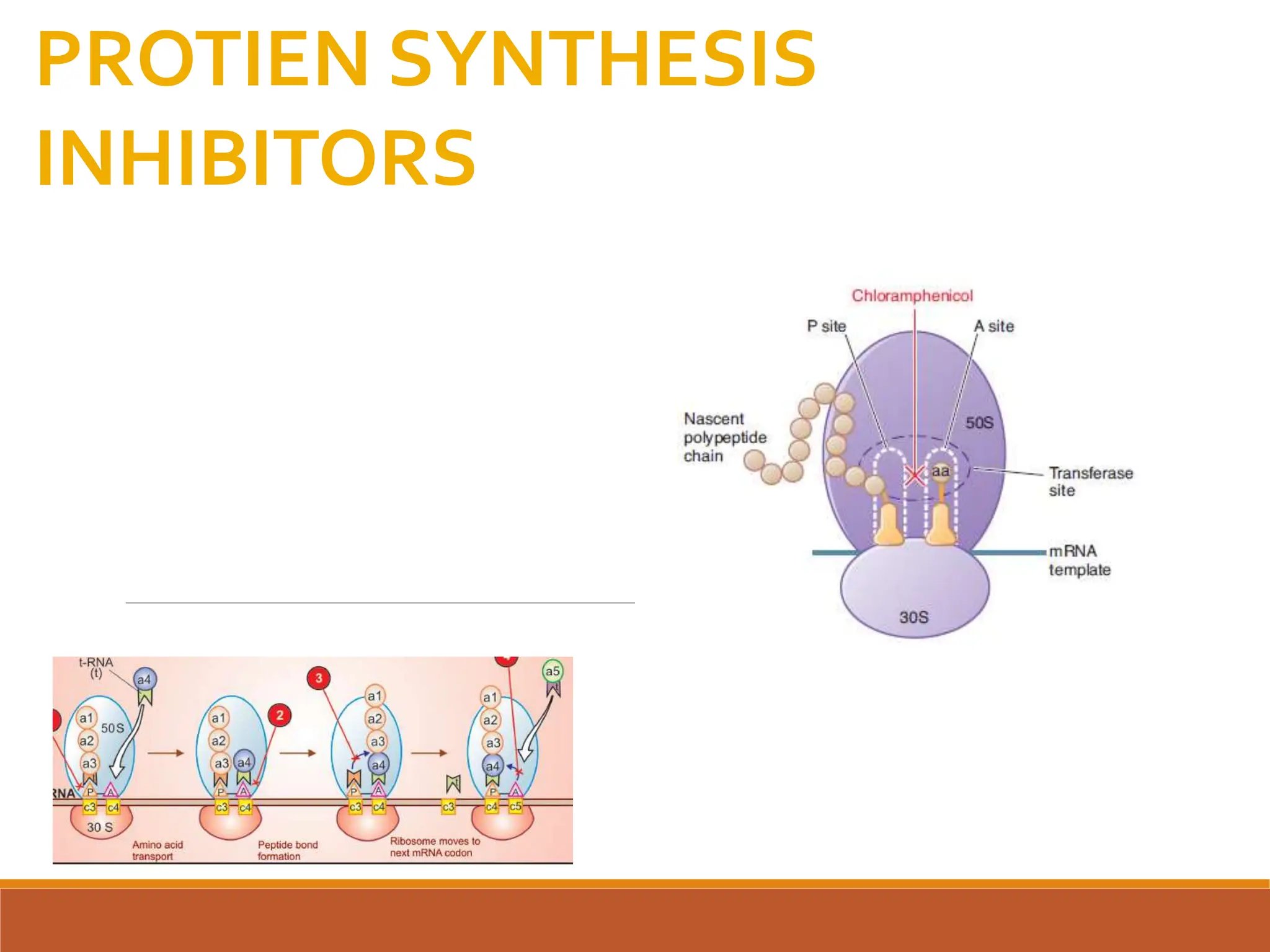
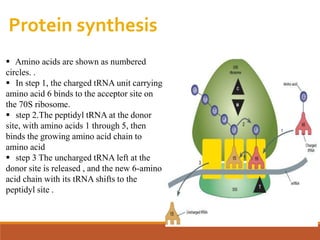
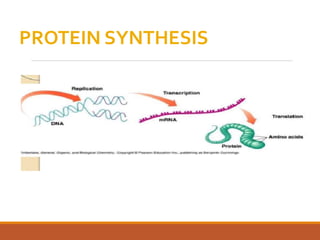
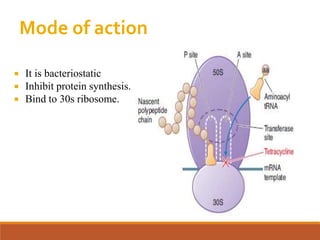
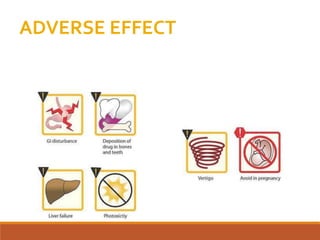
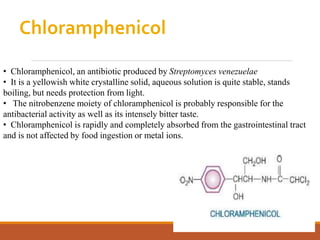
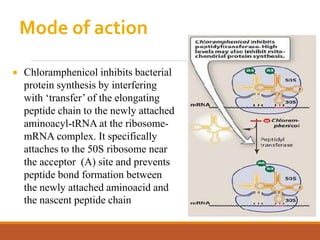
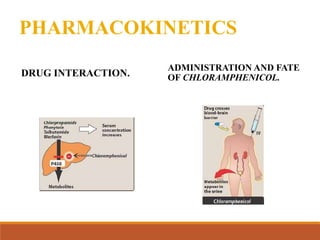
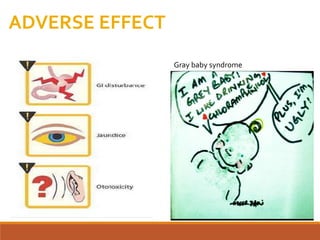
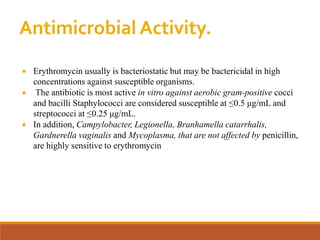
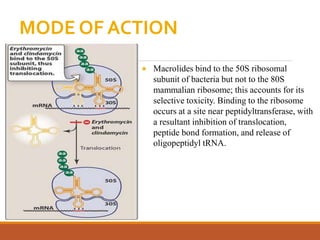
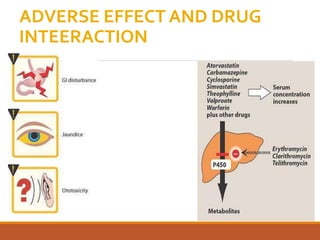
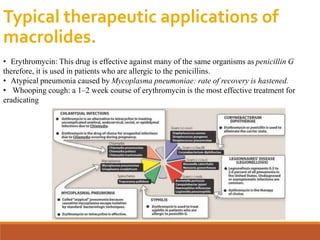
The document discusses protein synthesis and its inhibitors, detailing various antibiotics such as tetracyclines, chloramphenicol, and macrolides, along with their pharmacology, mechanisms of action, and resistance patterns. Tetracyclines are broad-spectrum antibiotics effective against various bacteria, while chloramphenicol is noted for its use in treating typhoid fever and macrolides like erythromycin are used for patients allergic to penicillin. The document emphasizes the selective antimicrobial action based on the structural differences between bacterial and mammalian ribosomes.