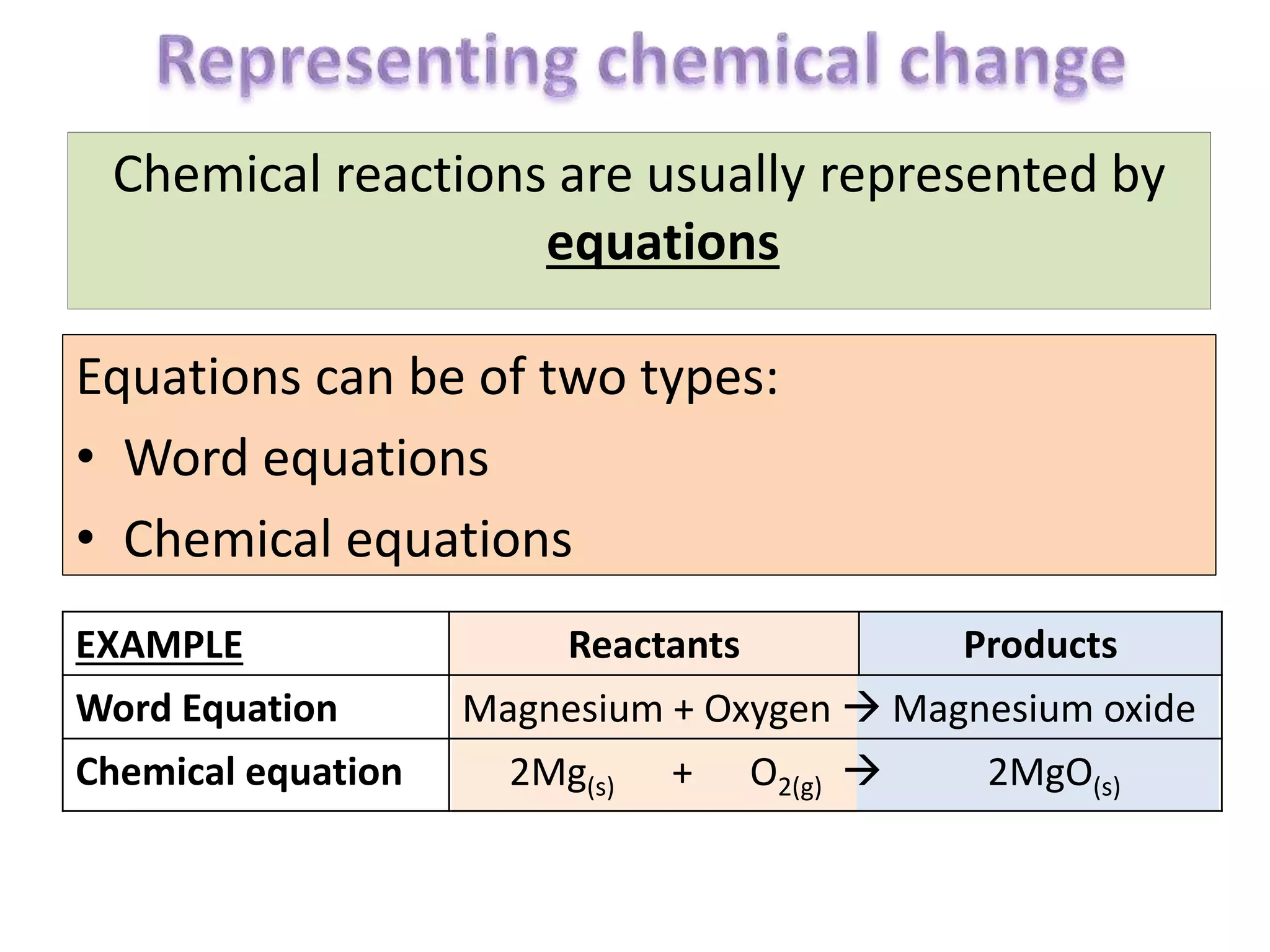
The document discusses chemical equations and balancing chemical reactions. It explains that chemical equations are used to represent chemical reactions and must be balanced so that the same number of atoms are on both sides of the reaction arrow. It provides examples of balancing various chemical equations by adding coefficients in front of chemical formulas like 2Na + Cl2 → 2NaCl. It also notes that state symbols should be included to indicate if reactants and products are solids, liquids, gases or aqueous.