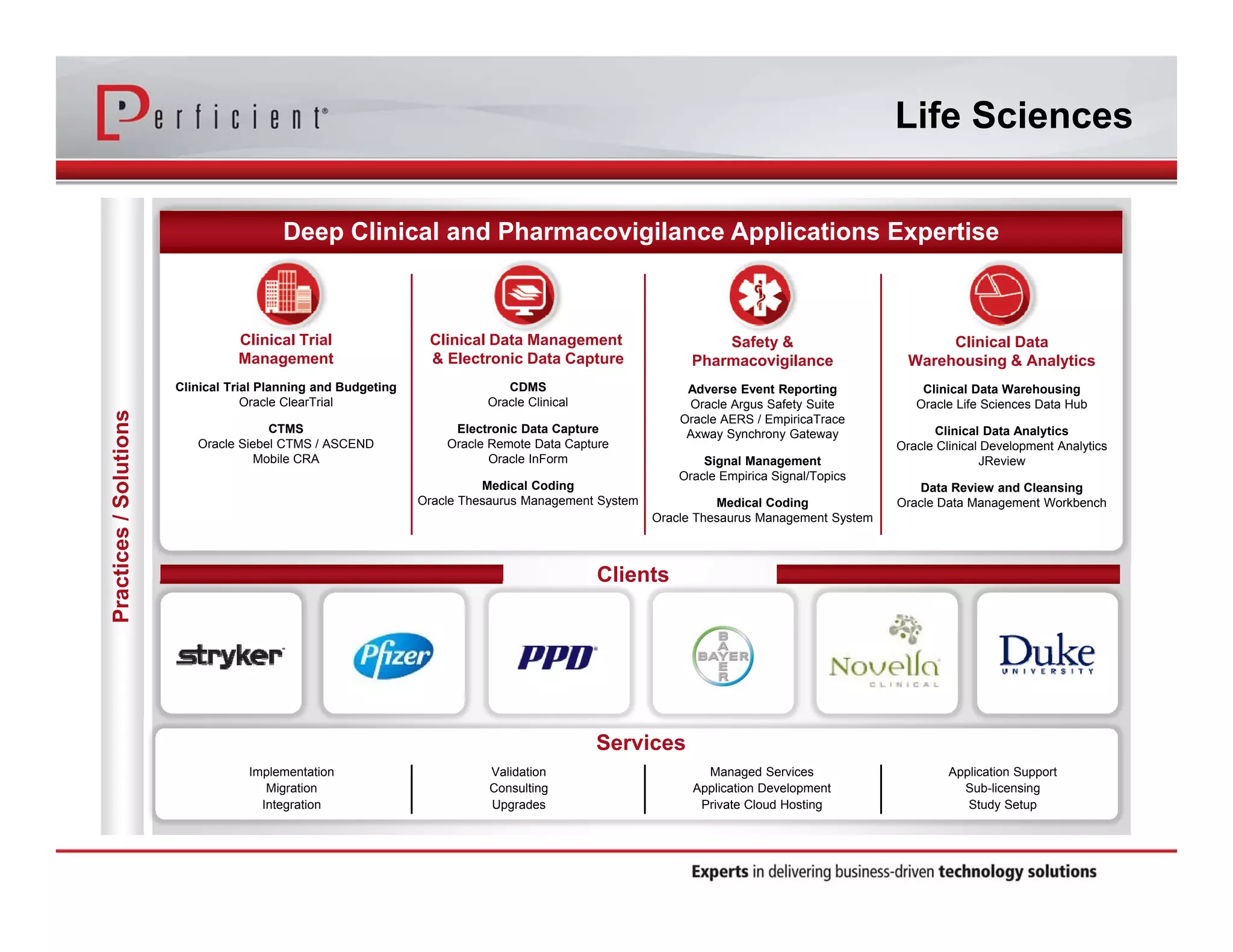
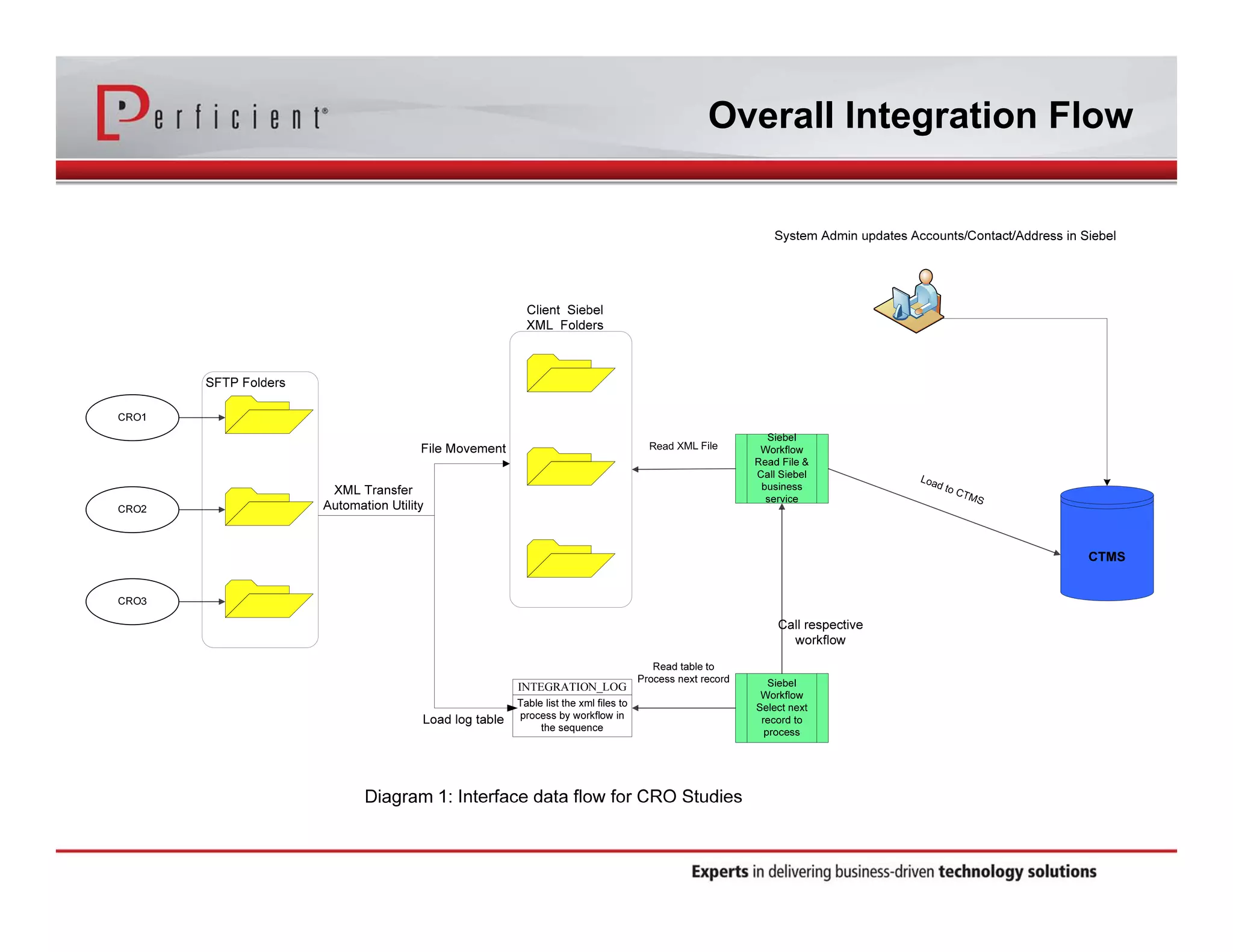
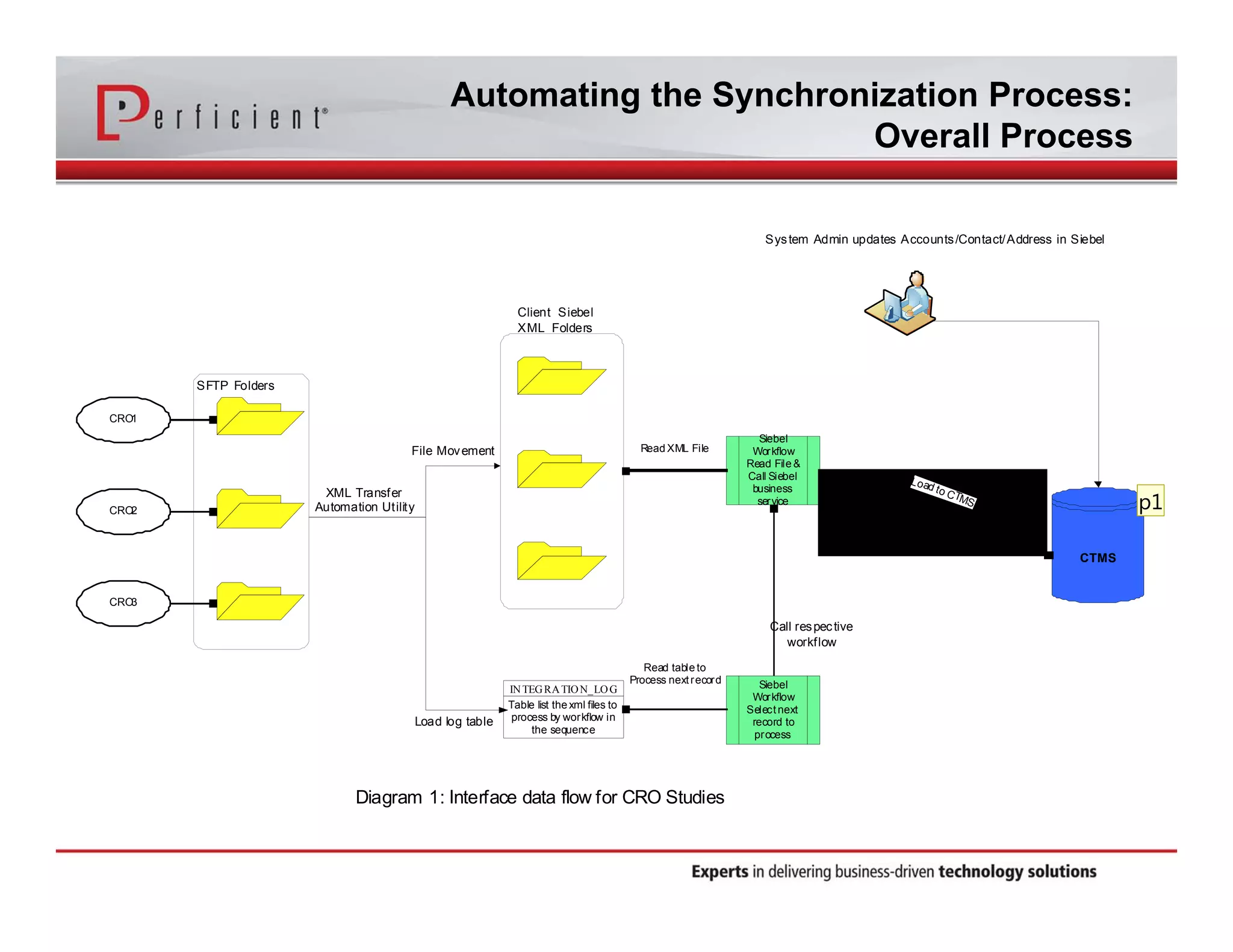
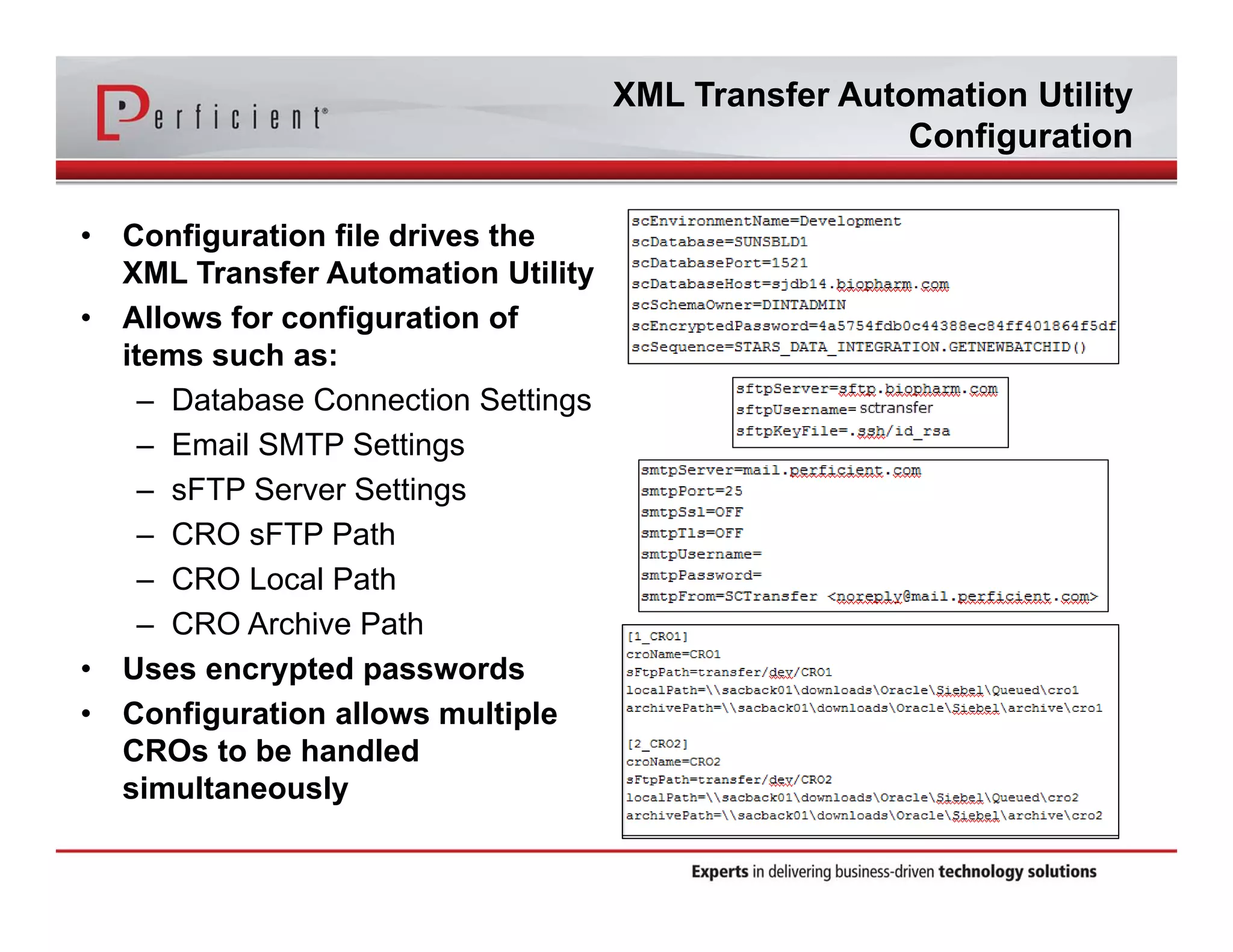
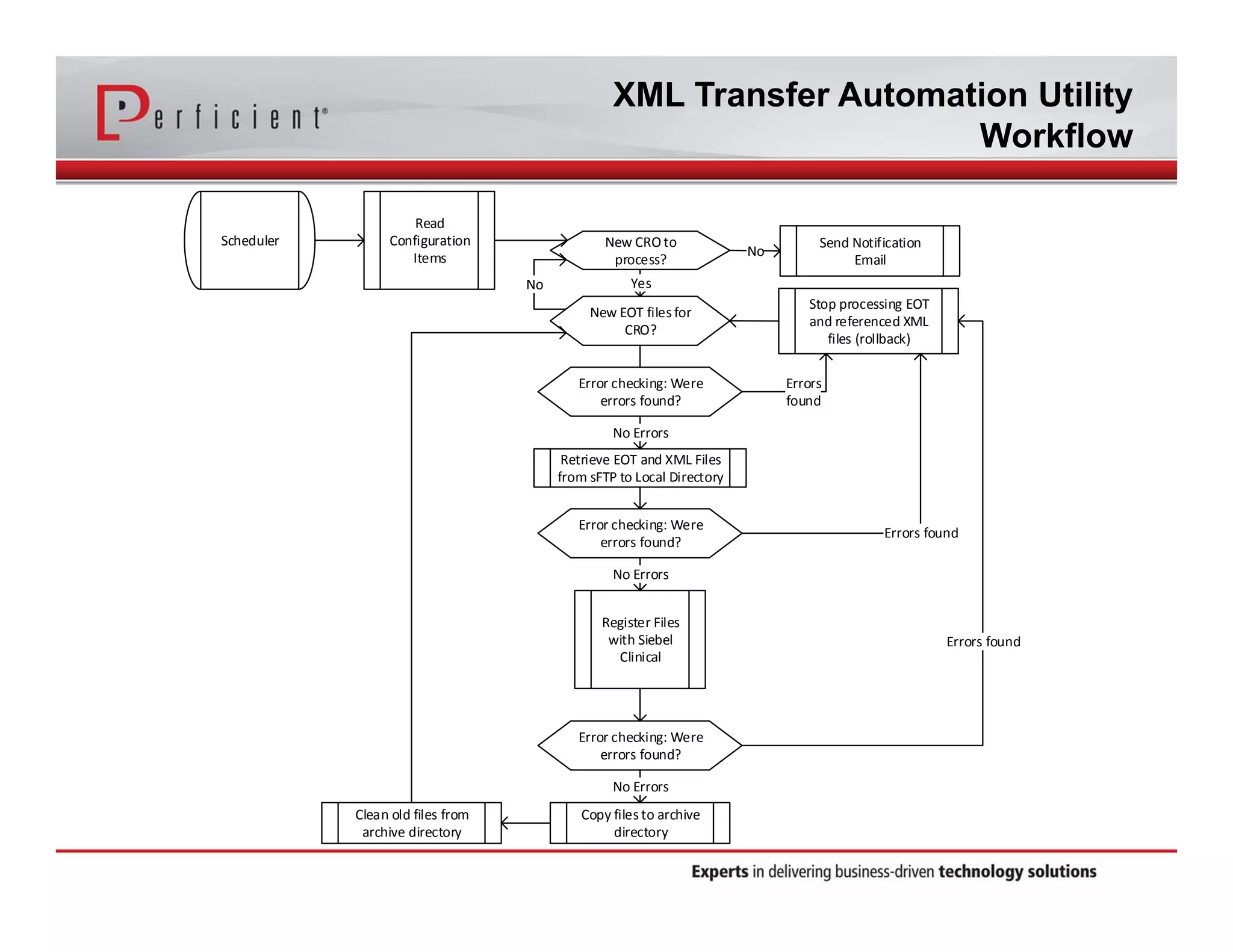
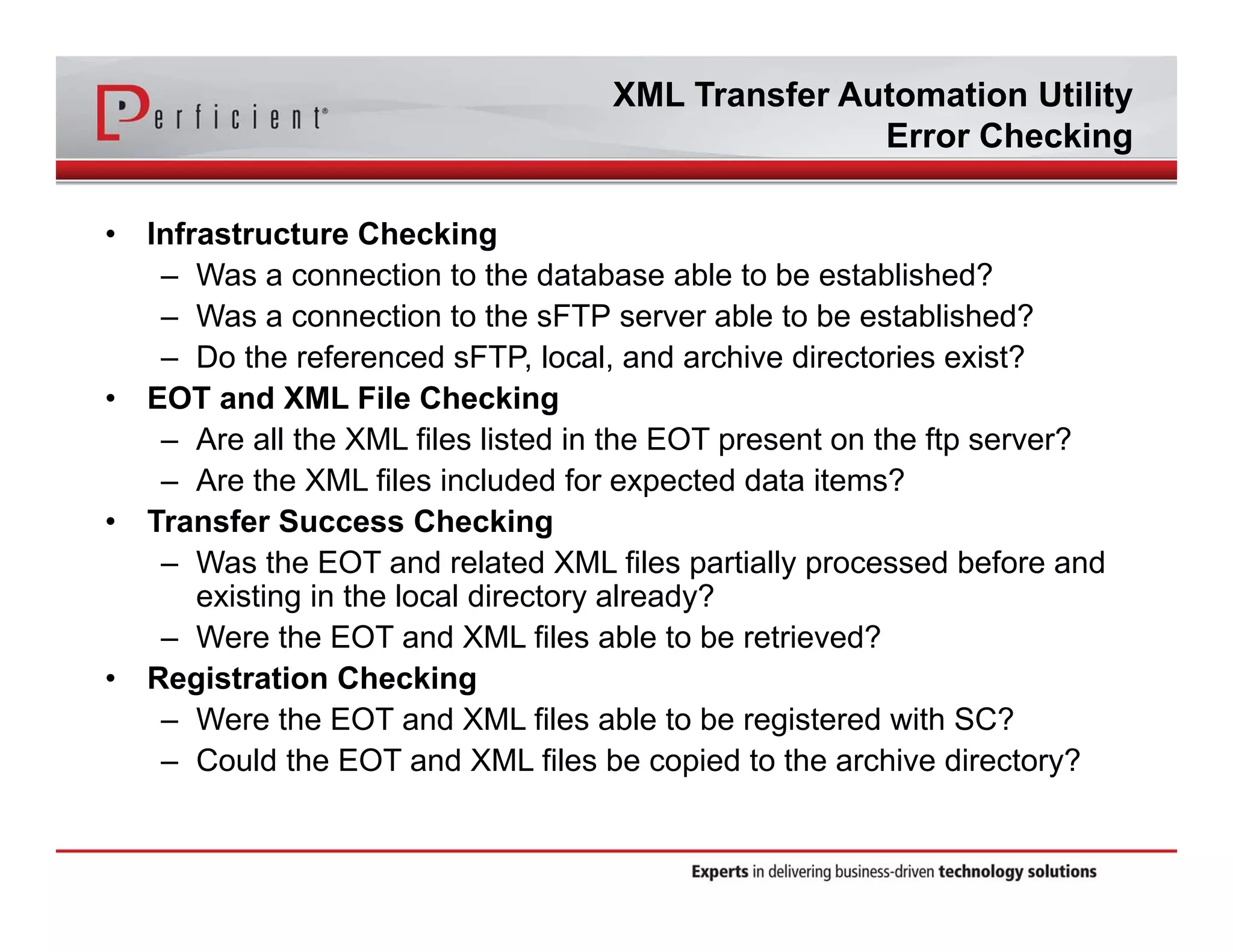
This document discusses automating the transfer of clinical trial management data from multiple contract research organizations (CROs) into an Oracle Siebel CTMS system. It describes transferring standardized XML files on a regular basis using an XML file transfer utility. This utility automatically retrieves and processes the XML files, performs error checking, and loads the data into the CTMS while logging activity. This allows clinical trial data from multiple CRO partners to be consolidated in a single system.