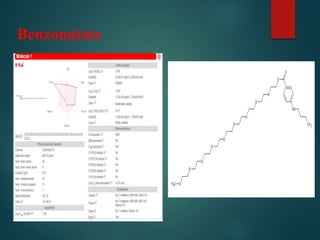
This document discusses various properties of antitussive compounds. It begins by explaining how antitussives work by either central or peripheral mechanisms to inhibit coughing. It then lists some example antitussive compounds that act centrally or peripherally. The document outlines a work plan to retrieve, draw, and analyze various antitussive molecules. Tables and graphs are presented that compare the molecules' properties such as molecular weight, hydrogen bonding, and Lipinski rule compliance. In conclusion, opium is identified as having high water solubility and bioavailability compared to the other antitussive compounds analyzed.





(C2=CC=CC=C2)[C@@H](C)CN(C)C
Clofedanol CN(C)CCC(C1=CC=CC=C1)(C2=CC=CC=C2Cl)O
Opium C1=C(OC(=C(C1=O)O)C(=O)O)C(=O)O
Homatropine CN1C2CCC1CC(C2)OC(=O)C(C3=CC=CC=C3)O
Phenylpropanolamine CC(C(C1=CC=CC=C1)O)N
Oxeladin CCC(CC)(C1=CC=CC=C1)C(=O)OCCOCCN(CC)CC
Pentoxyverine CCN(CC)CCOCCOC(=O)C1(CCCC1)C2=CC=CC=C2
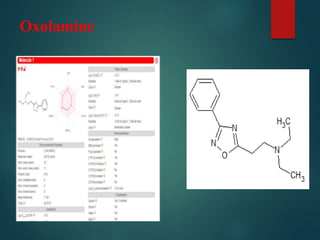
Oxolamine CCN(CC)CCC1=NC(=NO1)C2=CC=CC=C2](https://image.slidesharecdn.com/antitussivesdrugmolecules-191108071644/85/Antitussives-drug-molecules-6-320.jpg)



















![INTRENSIC WATER SOLUBILITY[LOGSW] VALUES FOR DIFFERENT
DRUGS
-10
-8
-6
-4
-2
0
SILICOS-IT LOGSW
-2.76
-6.64
-8.77
-2.45
-5.23
-5.68
-6.77
-5.92
-0.11
-2.53
-1.93
-5.97 -5.71
-5.12
Codeine Methadone Benzonatate Eucalyptol Noscapine
Pipazethate Levopropoxyphene Clofedanol Opium Homatropine
Phenylpropanolamine Oxeladin Pentoxyverine oxolamine](https://image.slidesharecdn.com/antitussivesdrugmolecules-191108071644/85/Antitussives-drug-molecules-28-320.jpg)






