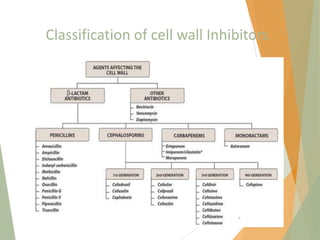
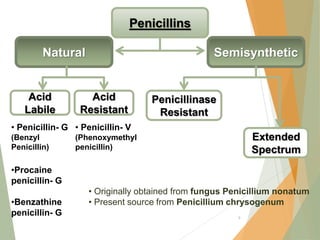
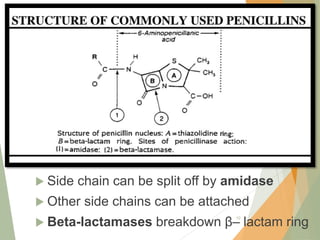
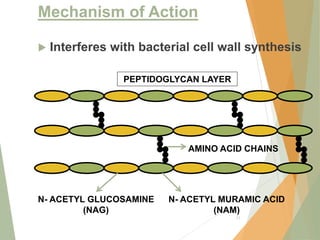
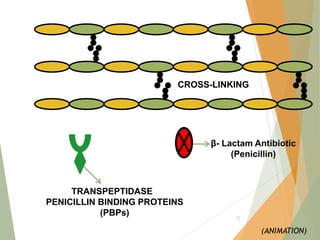
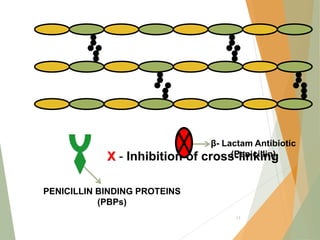
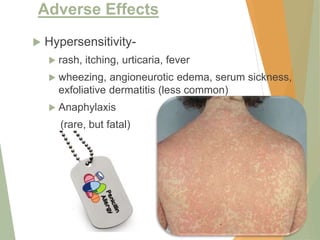
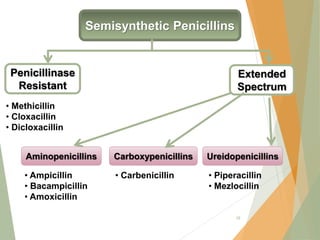
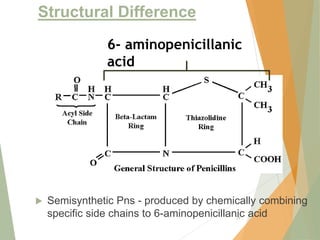
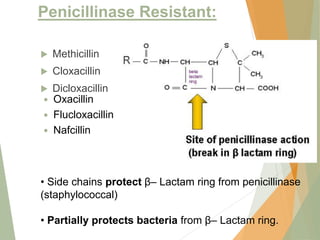
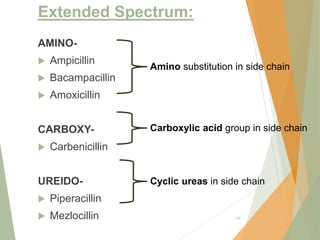
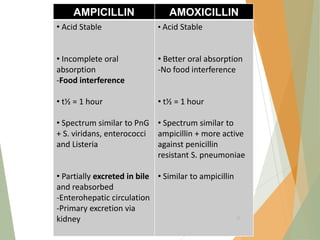
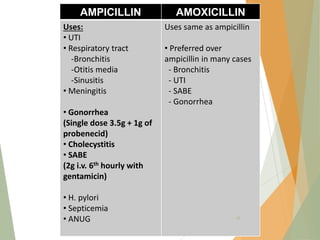
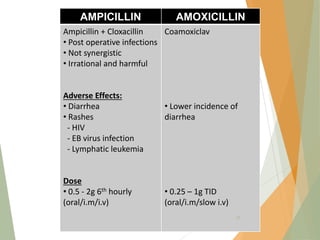
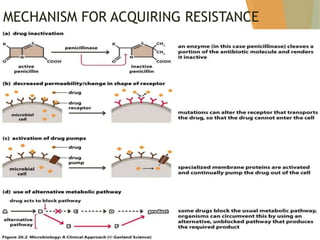
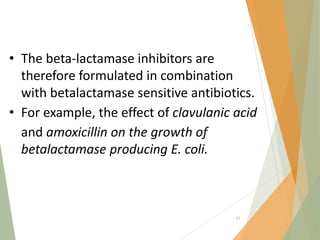
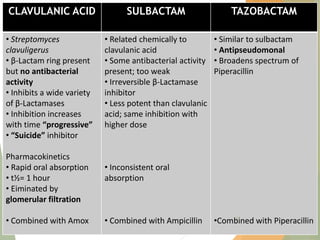
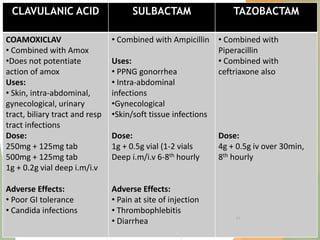
The document discusses antibiotics that target bacterial cell wall synthesis, specifically focusing on beta-lactam antibiotics such as penicillin and its derivatives. It covers the history, classification, mechanism of action, antibacterial spectrum, uses, and adverse effects associated with penicillin, including the development of resistance. It also briefly outlines other classes of antibiotics, such as cephalosporins and carbapenems, emphasizing their structural similarities and resistance mechanisms.