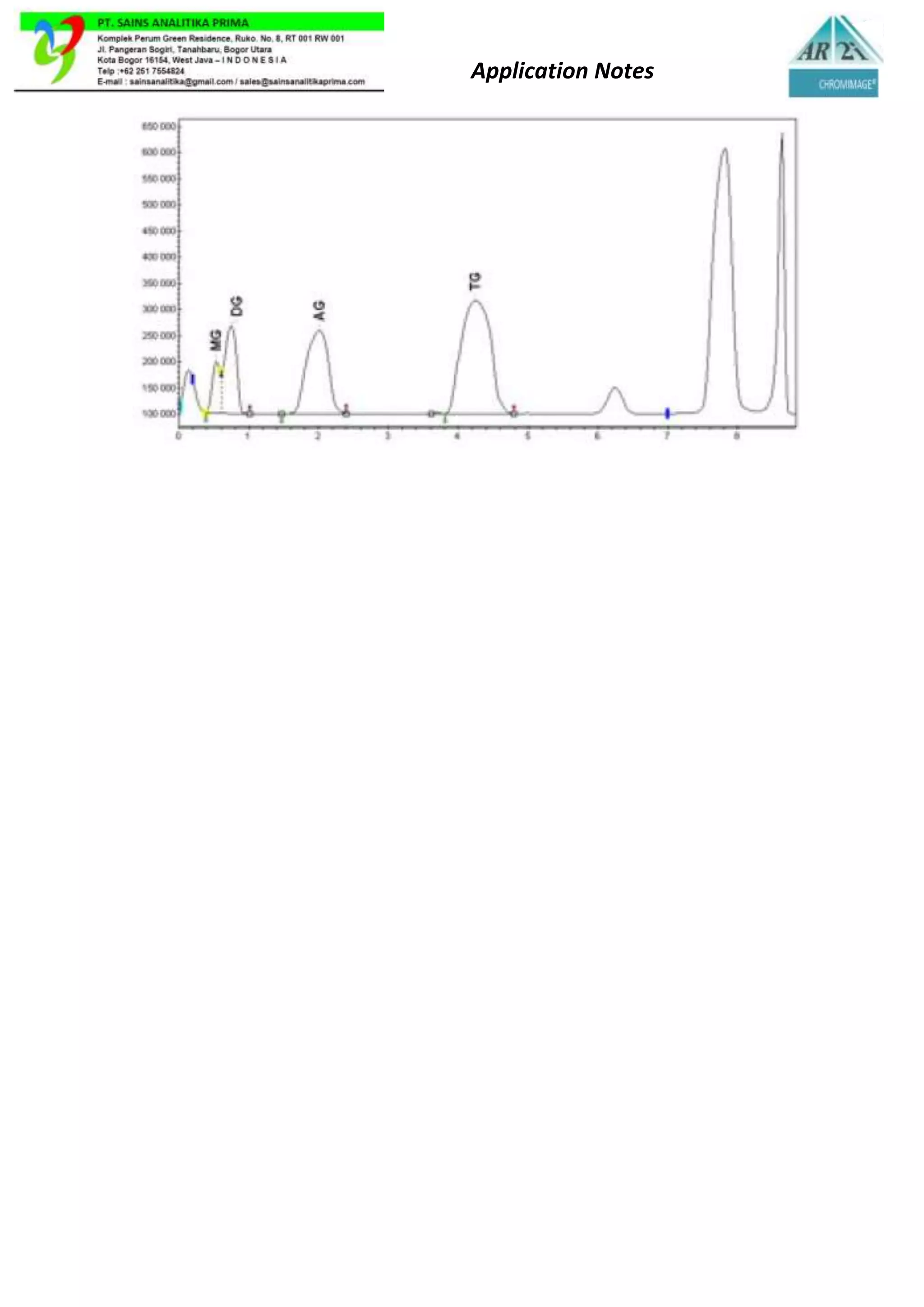
Thin layer chromatography (TLC) is a method used to separate components in a lipid mixture based on their polarity. TLC involves a stationary phase, usually a silica-coated plate, and a mobile phase of solvents. As the mobile phase travels up the plate, the lipid components migrate at different rates depending on their solubility and polarity. Less polar lipids migrate farther. TLC can identify unknown lipid components by comparing their migration distances to standards. It allows for faster analysis of small lipid amounts than column chromatography.

![Application Notes
Method
Chromatographic visualizer : AR2i CHROMIMAGE® 3X
Chromatographic plate : HPTLC silica 60, 10 x 20 cm
Phase mobile :
i. Petroleum ether 80 ml
ii. Diisopropyl ether 20 ml
iii. Acetic acid glacial 1 ml
Sample solutions :
1 mg/ml in monoglycerides, diglycerides and fatty acids
2 mg/ml in triglycerides
Solvent :
Chloroform / Methanol [2 vol./ 1 vol.]
Reagent addition :
i. Copper sulfate 10 g
ii. Purified water 69 ml
iii. Ethanol 24 ml
iv. Phosphoric acid at 85% 8 ml
Heat 120 minutes at 105°C
Detection :
In the visible: Black spots on a bluish background.](https://image.slidesharecdn.com/an-sap01-ar2ilipidsanalysis-221115085616-885cb249/75/AN-SAP01-AR2I-Lipids-analysis-docx-2-2048.jpg)