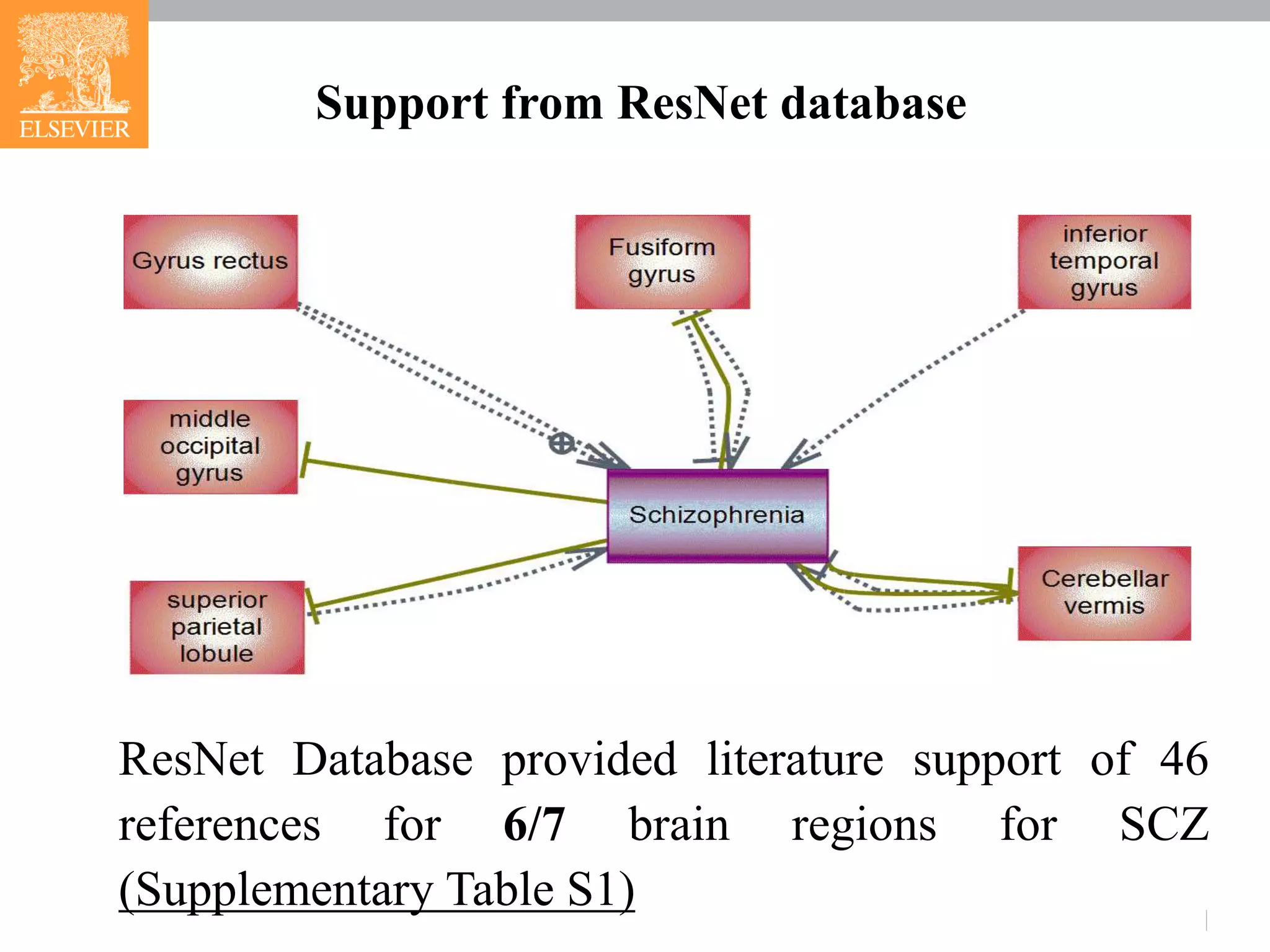
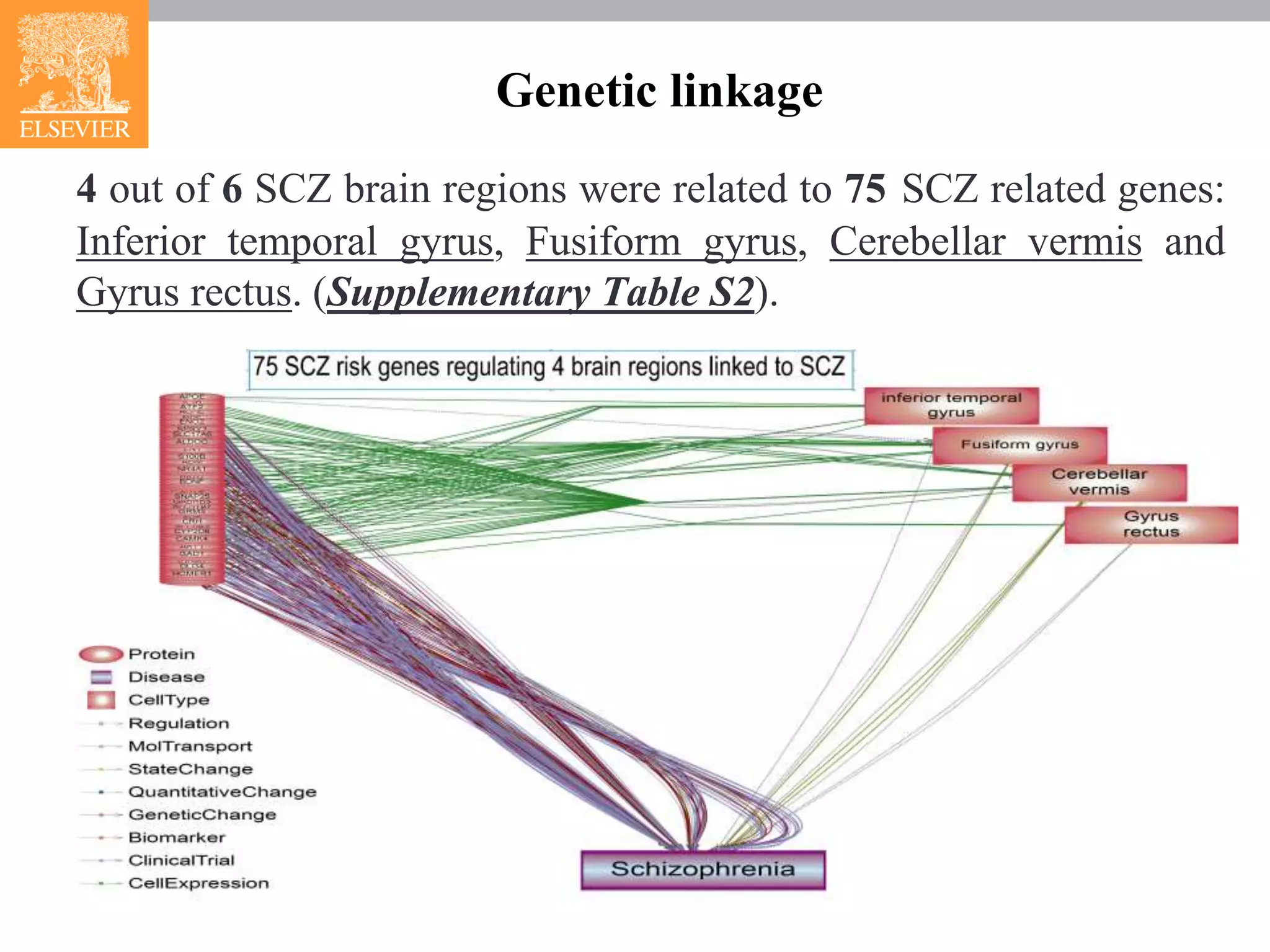
The document presents research on schizophrenia (scz), identifying genetic and brain region pathways contributing to its pathogenesis using functional magnetic resonance imaging (fMRI) and a brain-gene ResNet database. Analysis revealed connectivity features associated with specific brain regions and suggested three novel genes (pten, fgf8, cd38) linked to scz. The integration of fMRI data with genetic information advances understanding of scz mechanisms and potential therapeutic targets.

![fMRI data
functional Magnetic Resonance Imaging (fMRI) data set
Data were collected with 1.5 T GE MRI scanner;
Participants were from Kunming, China;
All 32 patients were treatment resistant;
All 31 healthy siblings had no schizophrenia history or related symptoms.
Imaging Data Preprocessing
SPM8 and REST [1] toolbox: realigned, re-sliced, normalized, band-pass filtered
(0.01~0.08 Hz) and smoothed (3-D Gaussian kernel with 6mm FWHM).
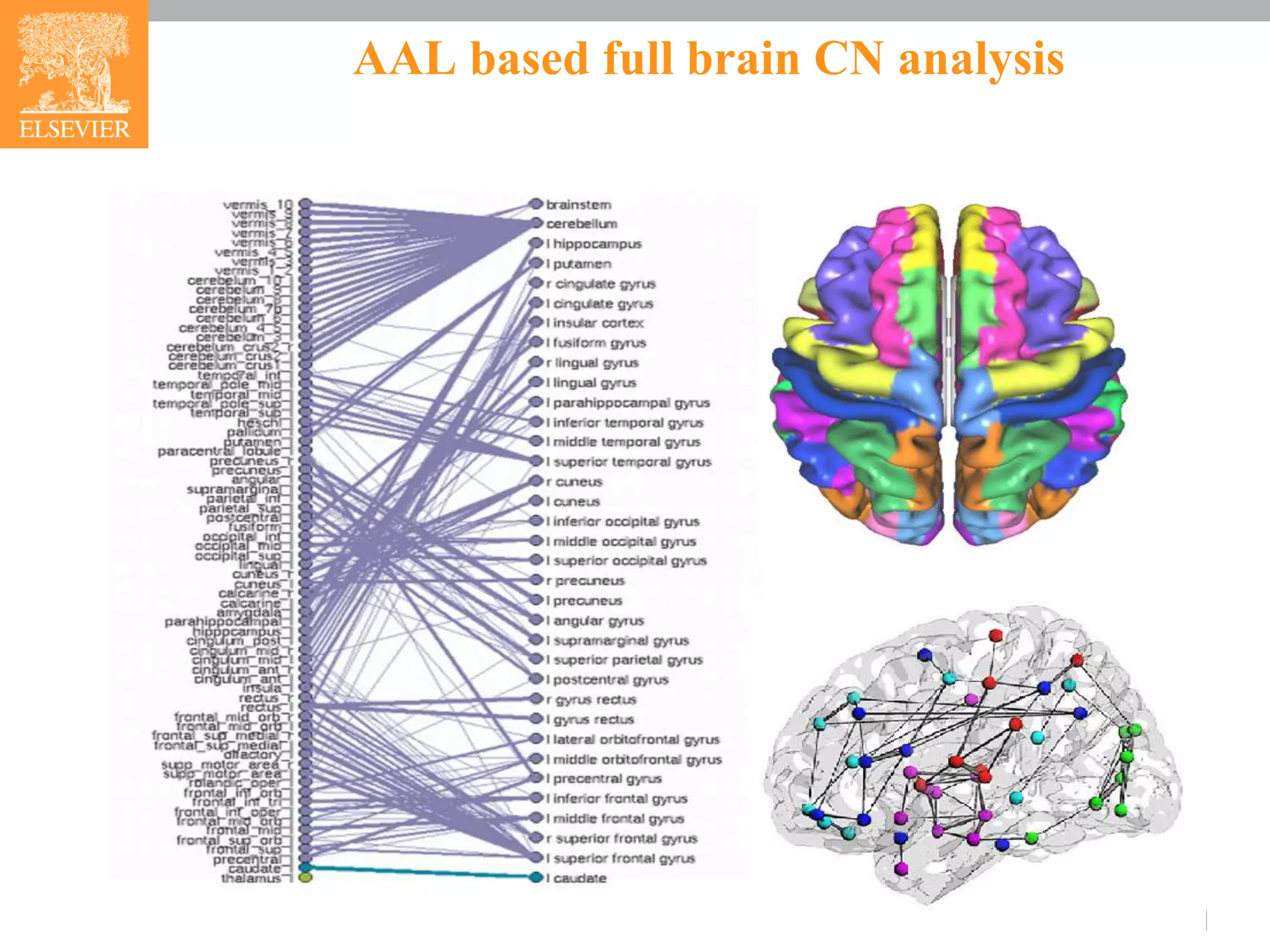
Whole brain mask was acquired using WFU_PickAtlas toolbox, containing the 116 AAL
brain regions [2].](https://image.slidesharecdn.com/fmrianalysisinps-180607164949/75/Analysis-of-Functional-Magnetic-Resonance-Imaging-fMRI-data-from-human-brain-in-Pathway-Studio-3-2048.jpg)
![Brain-Gene Resnet (BGR) data in Pathway Studio
Pathway Studio ResNet ® Mammalian database
http://pathwaystudio.gousinfo.com/ResNetDatabase.html
Real-time updated network databases, including curated signaling,
cellular processes and metabolic pathways, ontologies and
annotations, molecular interactions and functional relationships.
Have been widely used to study modeled relationships between
proteins, genes, complexes, cells, tissues and diseases
http://pathwaystudio.gousinfo.com/Mendeley.html
The largest literature based network database among known
competitors in the field [3].](https://image.slidesharecdn.com/fmrianalysisinps-180607164949/75/Analysis-of-Functional-Magnetic-Resonance-Imaging-fMRI-data-from-human-brain-in-Pathway-Studio-4-2048.jpg)
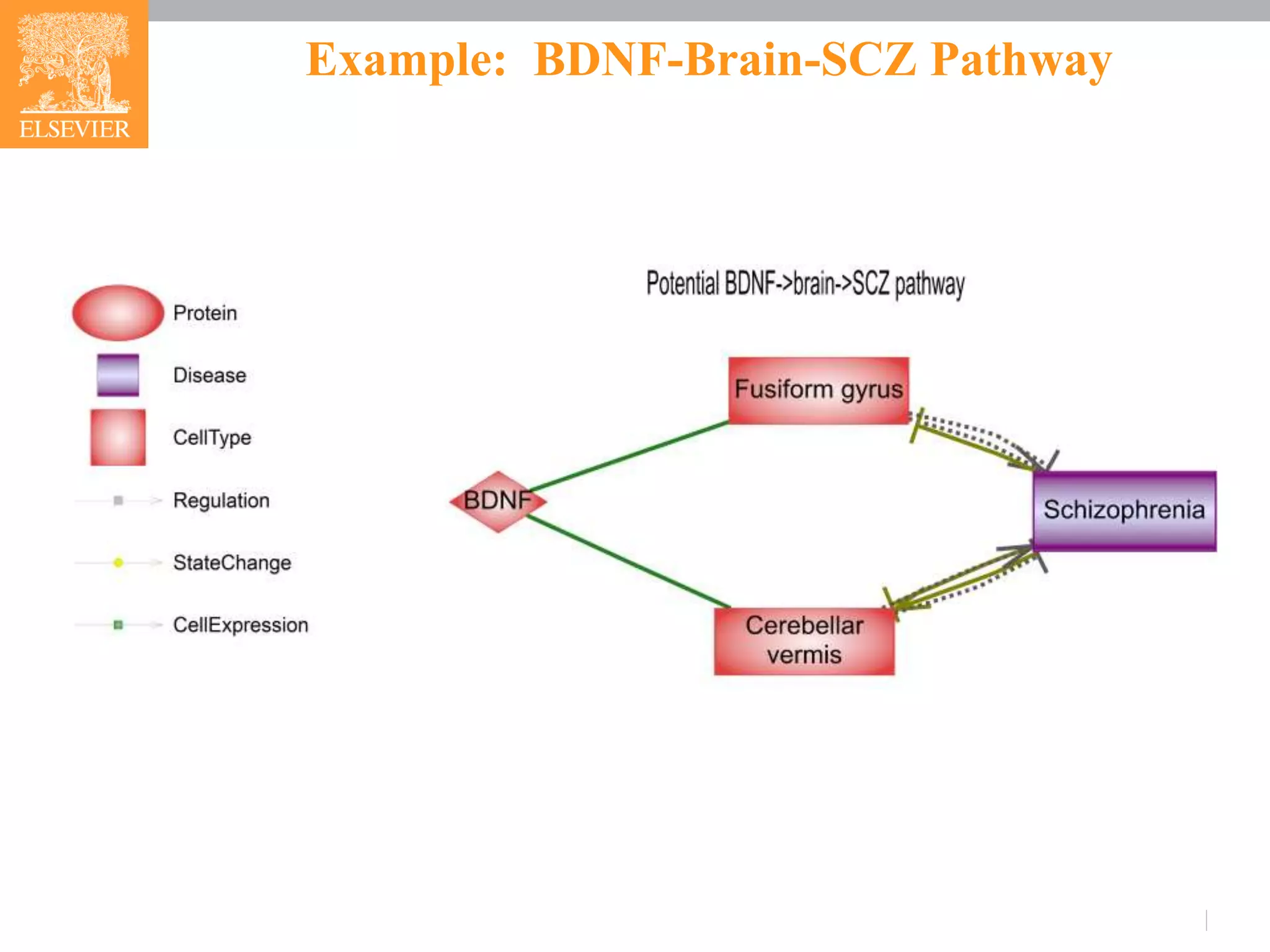
![Example: BDNF-Brain-SCZ Pathway
BDNF->Fusiform gyrus-> SCZ
Distortion of the balance among the 3 BDNF isoforms (pro-BDNF,
truncated BDNF and mature BDNF) could lead to changes in
connectivity and synaptic plasticity and, hence, behavior of
Fusiform gyrus [4].
Deficits in the fusiform gyrus is suggested as a trait pathology in
SCZ patients [5,6].
BDNF-> Cerebellar vermis-> SCZ
Decreased cerebellar BDNF mRNA and protein level could lead to
motoric impairment and Purkinje cell loss that damage cerebellar
vermis [7].
Cerebellar vermis is nominated a potential therapeutic target for the
treatment of SCZ [8, 9].](https://image.slidesharecdn.com/fmrianalysisinps-180607164949/75/Analysis-of-Functional-Magnetic-Resonance-Imaging-fMRI-data-from-human-brain-in-Pathway-Studio-11-2048.jpg)
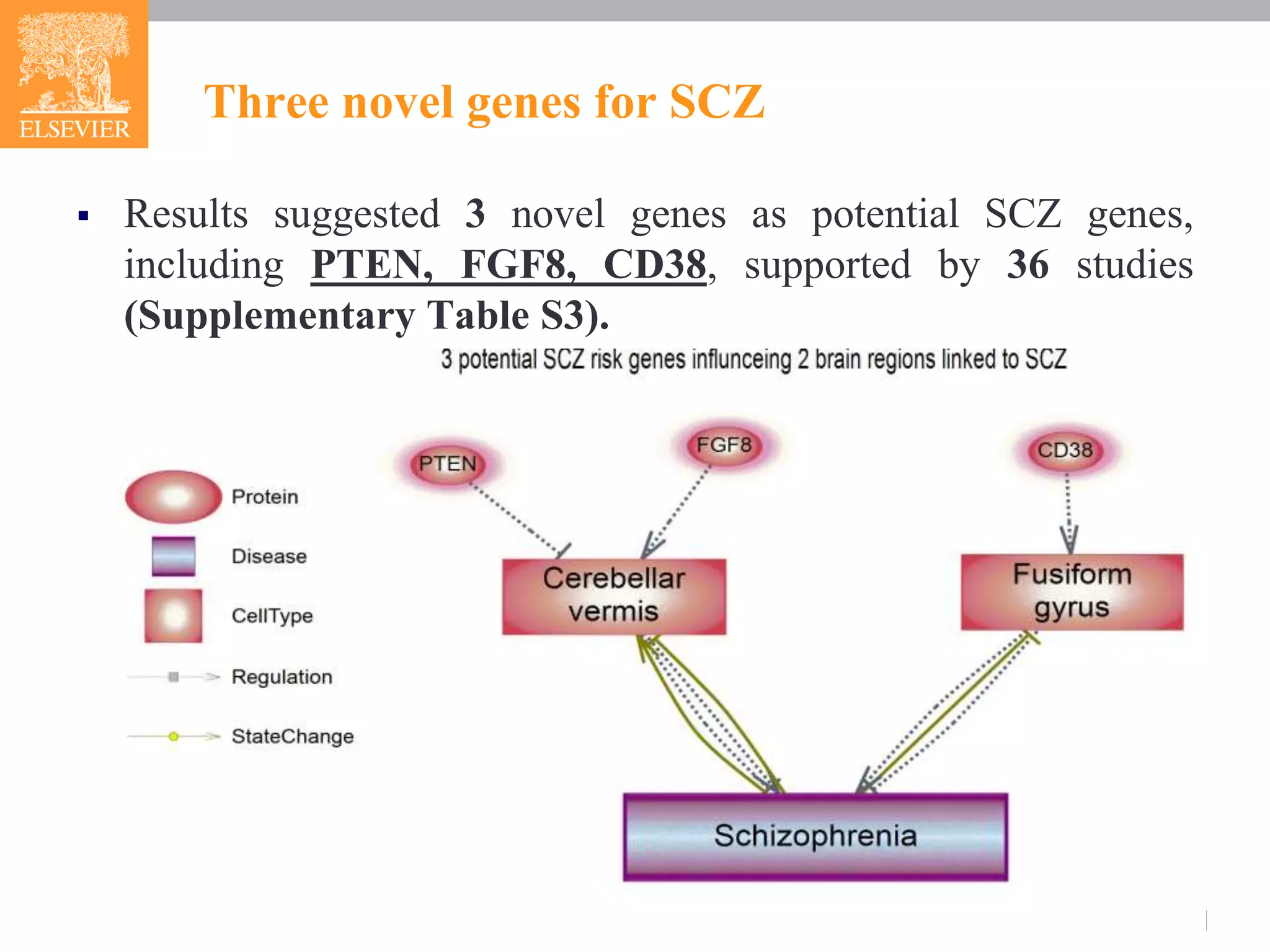
![Three novel genes for SCZ
CD38->Fusiform gyrus->SCZ
CD38 genotype is a genetic factor influencing the function of
Fusiform gyrus[10].
PTEN->Cerebellar vermis->SCZ
Deficiency of PTEN could lead to the loss of neurons and tau
hyperphosphorylation in cerebellar vermis [11].
FGF8 ->Cerebellar vermis->SCZ
Mutations in the FGF8 signaling pathway preferentially affect
the growth of the cerebellar vermis [12].](https://image.slidesharecdn.com/fmrianalysisinps-180607164949/75/Analysis-of-Functional-Magnetic-Resonance-Imaging-fMRI-data-from-human-brain-in-Pathway-Studio-13-2048.jpg)

![References
[1] Song, X., et al. PLoS.One. 2011; 6(9): 1-12.
[2] Tzourio-Mazoyer N, et al., Neuroimage. 2002; 15(1):273-89.
[3] Lorenzi PI et al. Autophagy. 2014; 10(7):1316-26.
[4] Kim DW, et al. Schizophr Res. 2013;151(1-3):165-74.
[5] Walther S, et al. Psychiatry Res. 2009;172(3):184-91.
[6]Choudhary M, et al. Schizophr Res. 2015 ;162(1-3):103-7.
[7] Firozan B, et al. Eur J Pharmacol. 2014;732:1-11.
[8] Villanueva R. Psychiatry Res. 2012;198(3):527-32.
[9] Garg S, et al. Psychiatry Res. 2016;243:413-20.
[10] Sauer C, et al. Neuropsychopharmacology. 2012;37(6):1474-82.
[11] Nayeem N, et al. Mol Cell Neurosci. 2007 Mar;34(3):400-8.
[12] Wen J, et al. PLoS One. 2013;8(5):e64451.](https://image.slidesharecdn.com/fmrianalysisinps-180607164949/75/Analysis-of-Functional-Magnetic-Resonance-Imaging-fMRI-data-from-human-brain-in-Pathway-Studio-16-2048.jpg)