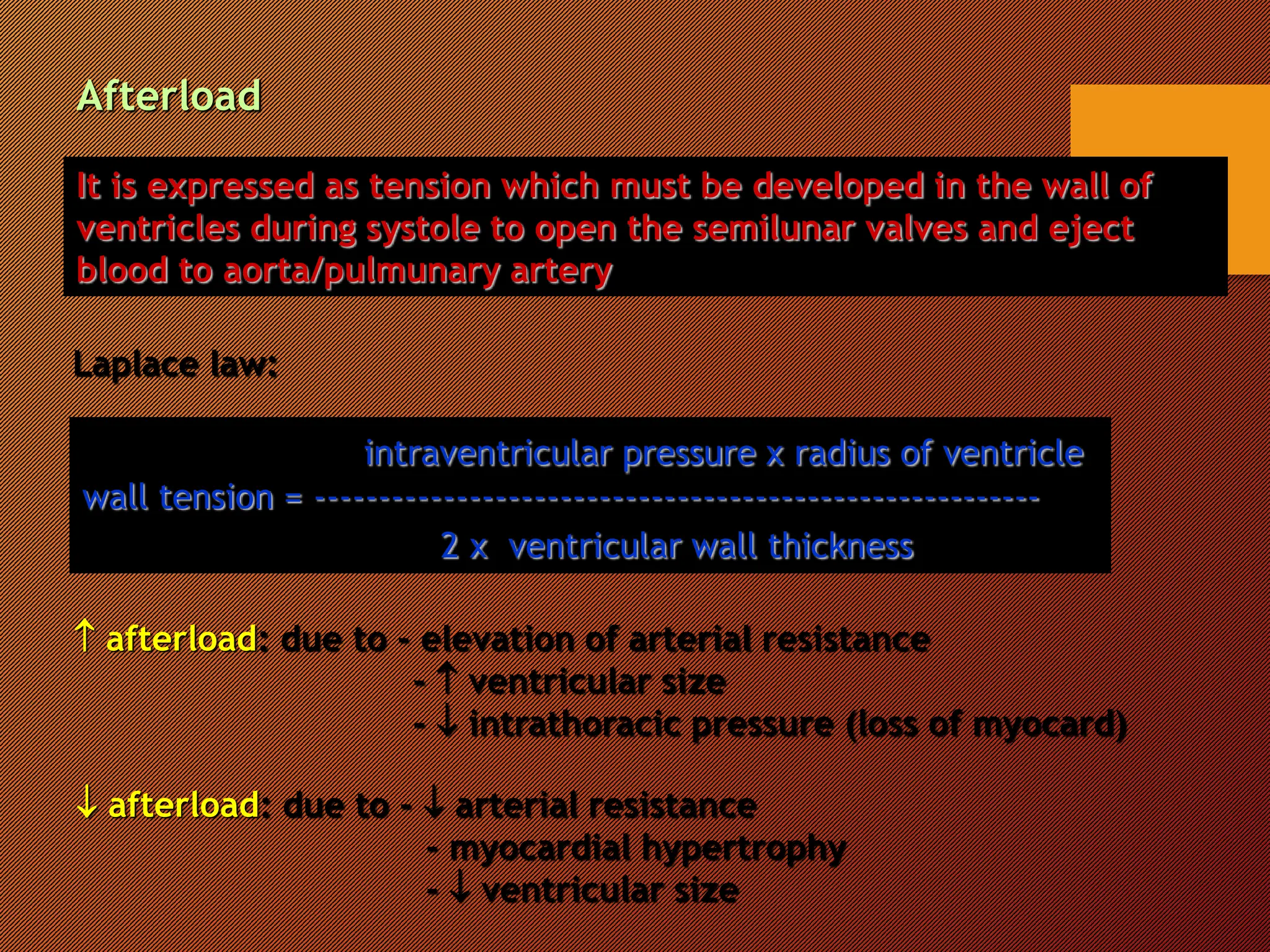
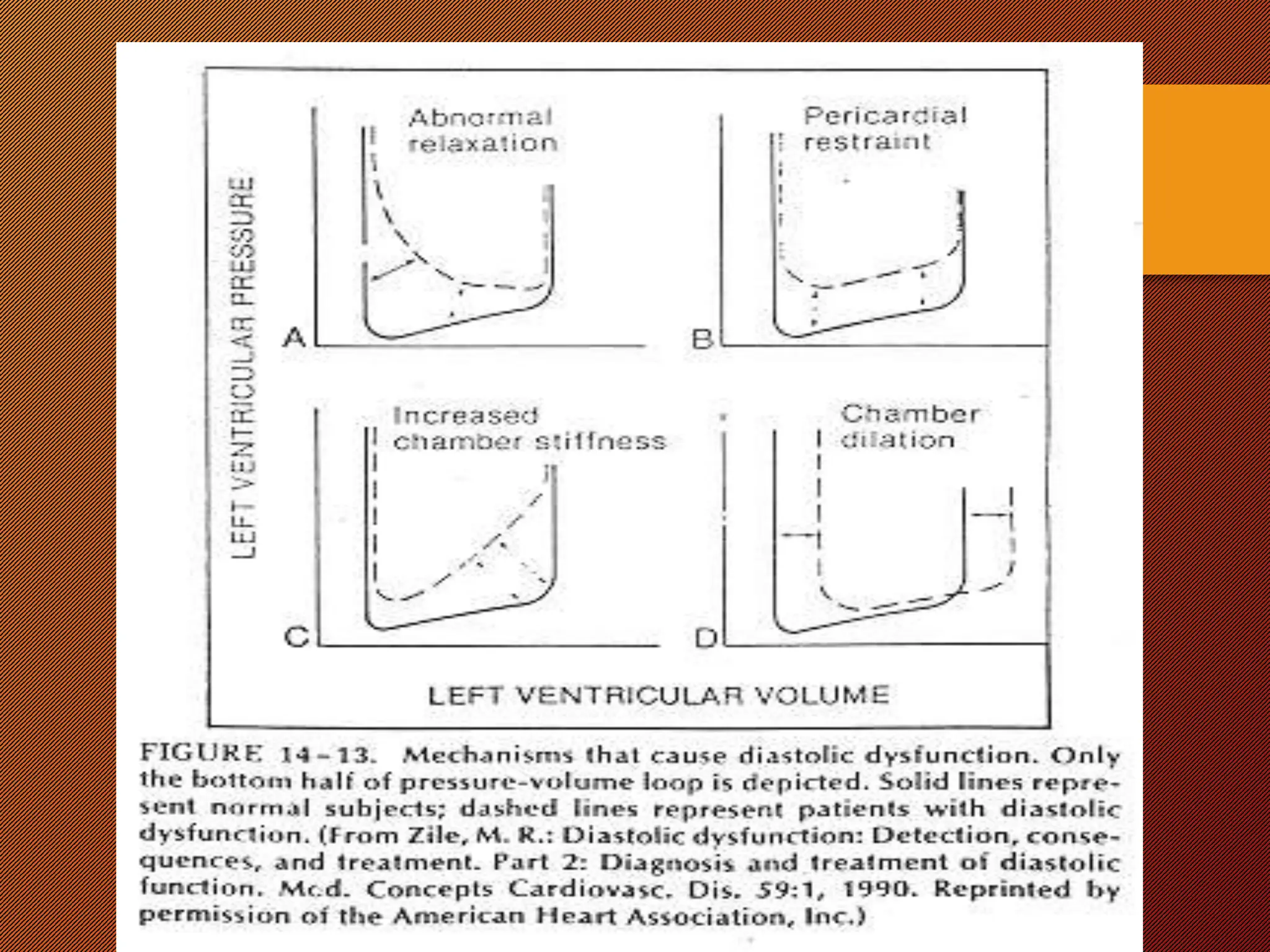
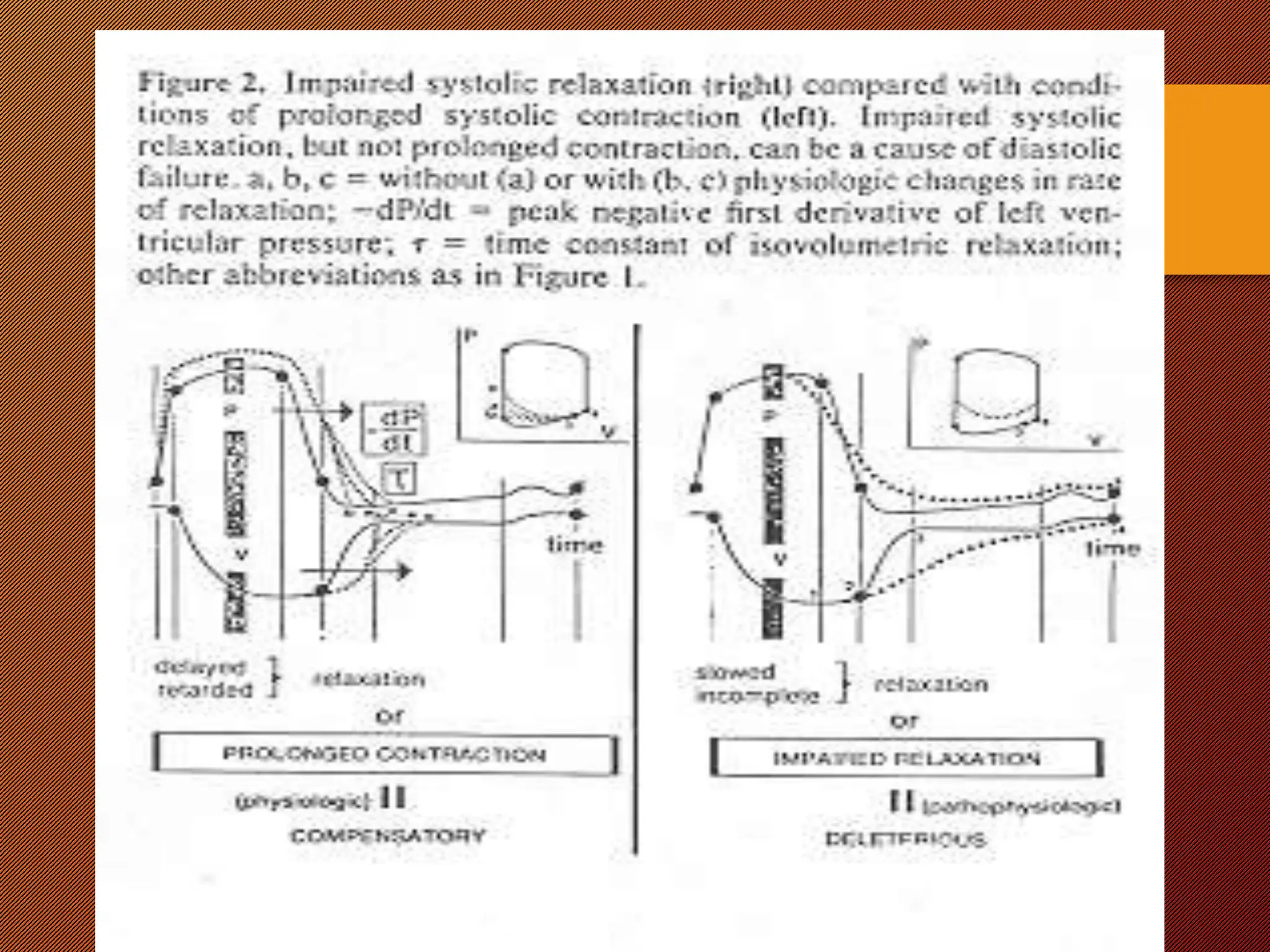
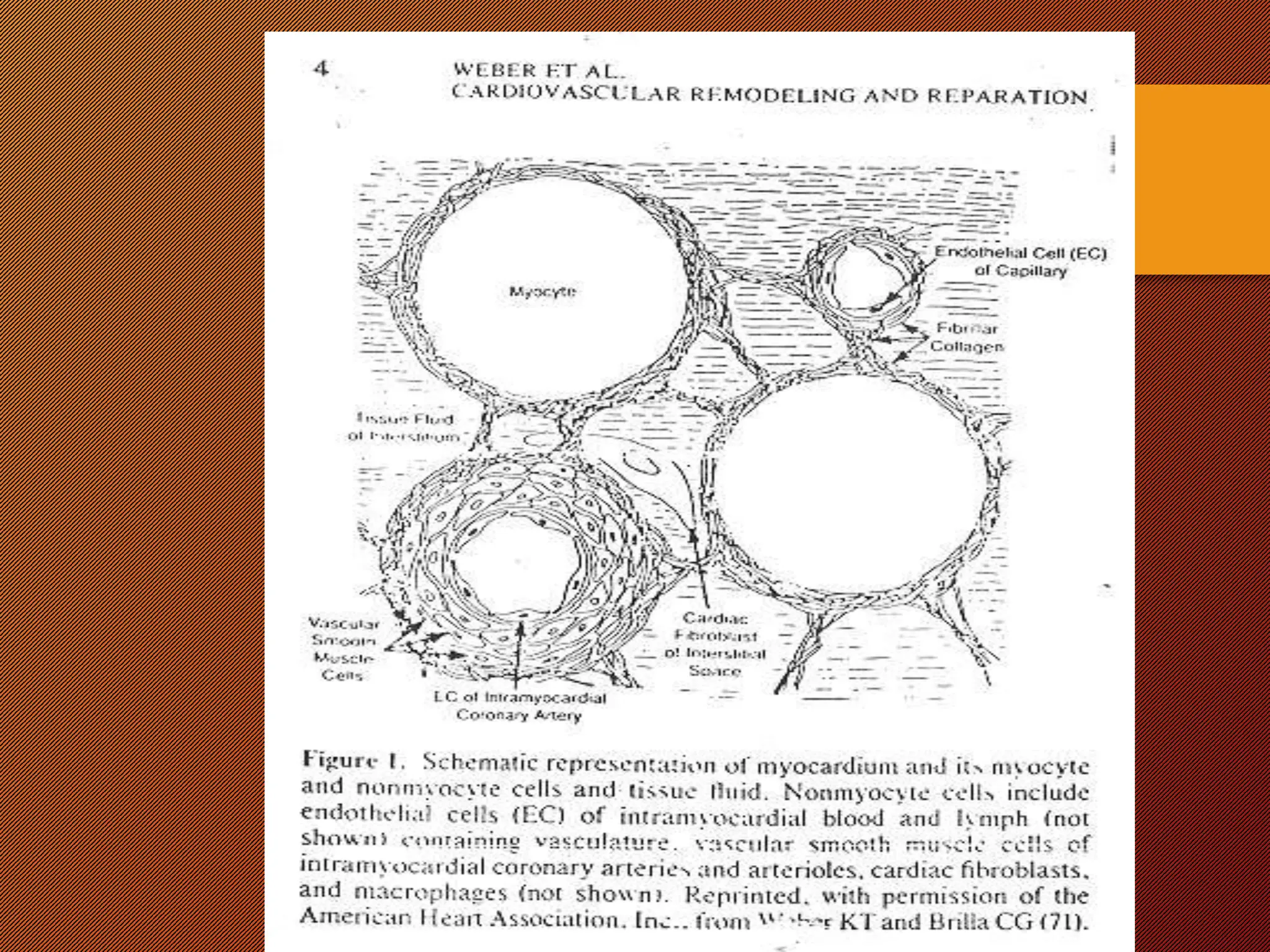
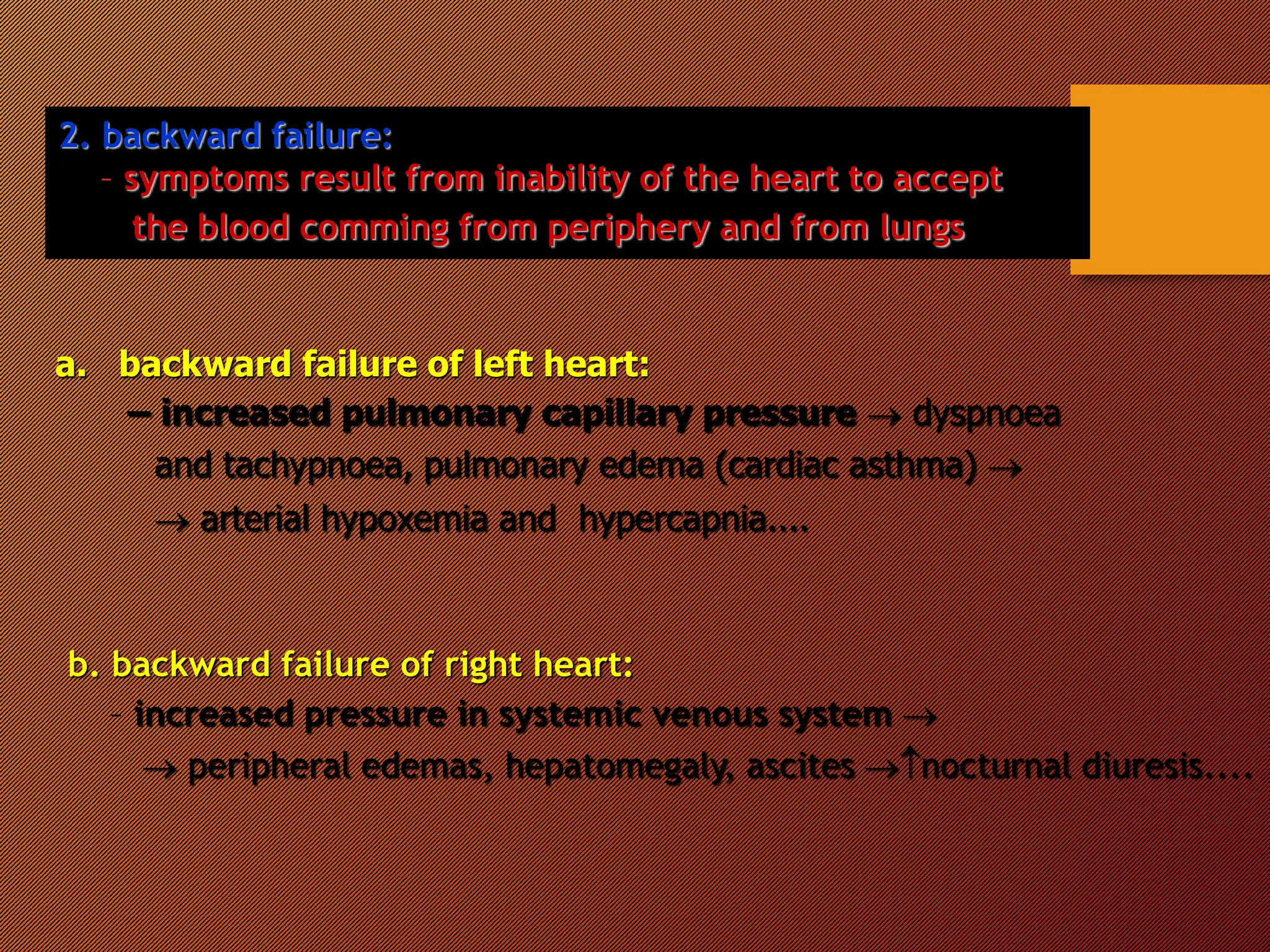
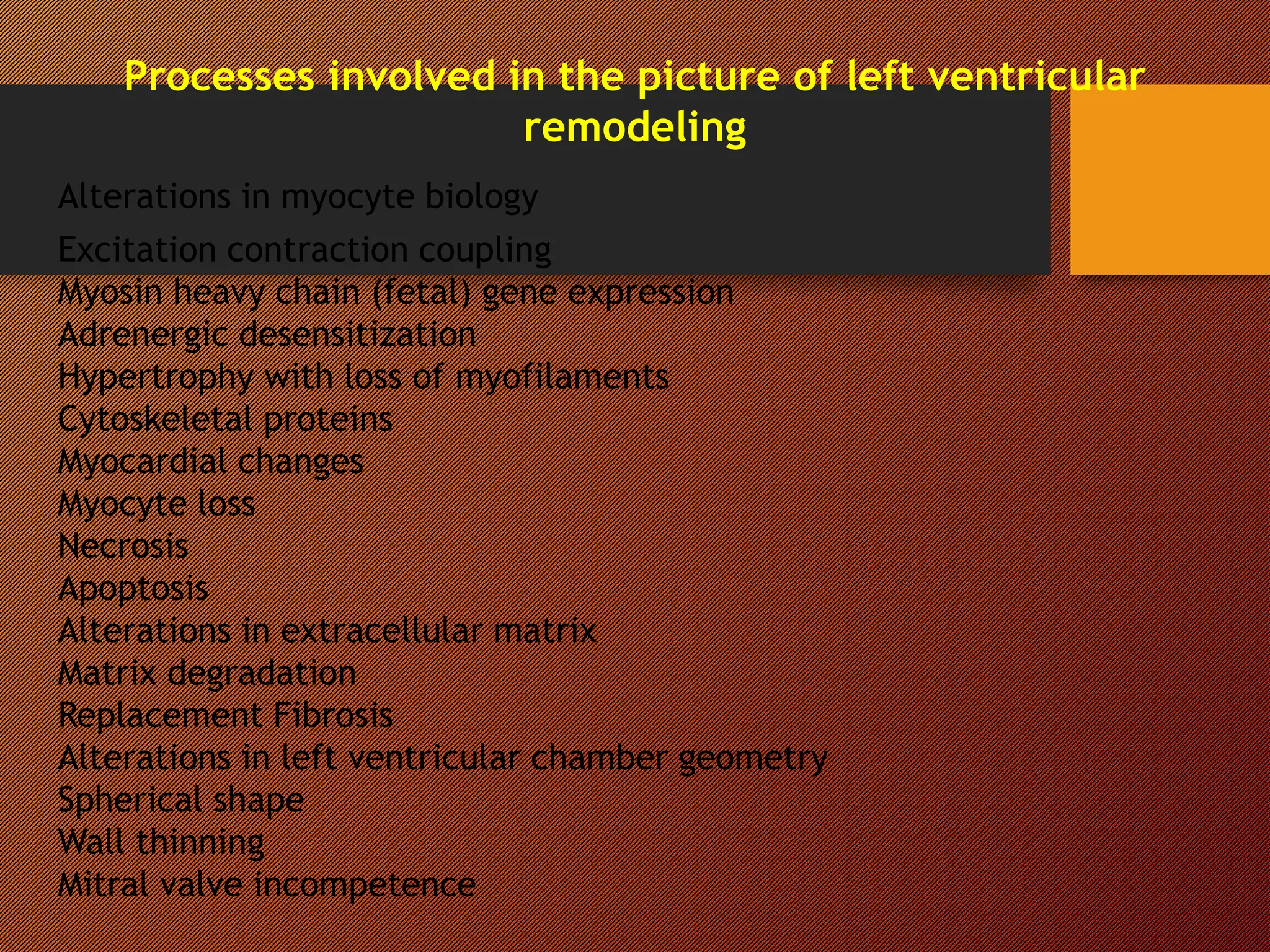
This document discusses the pathophysiology of heart failure. It begins by describing changes in myocardial contractility and the pressure-volume loop. Neurohumoral mechanisms are activated in heart failure as compensatory responses but become maladaptive over time, involving the sympathetic nervous system, renin-angiotensin-aldosterone system, and other factors. Left ventricular remodeling further worsens the heart's mechanical function and efficiency. Both systolic and diastolic dysfunctions can occur and are characterized by different structural and functional features.