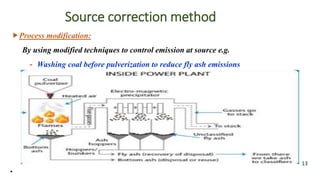
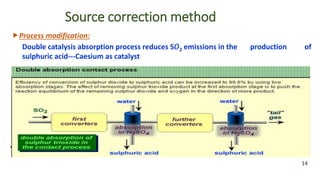
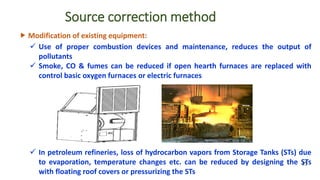
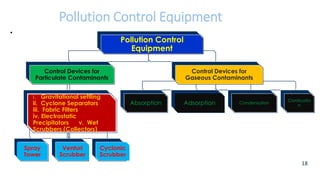
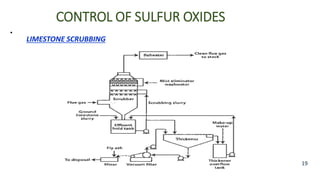
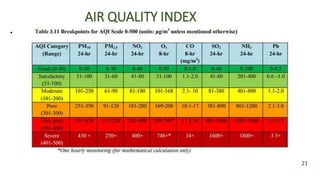
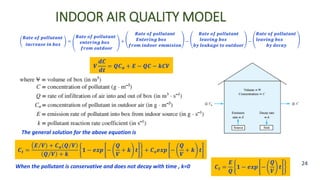
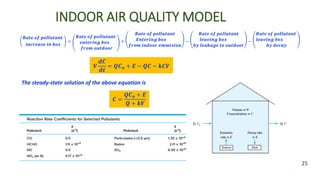
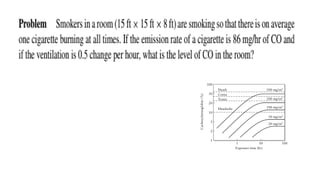
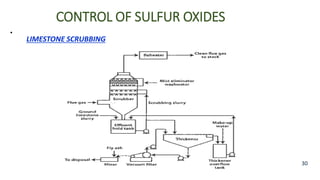
The document discusses air pollution, including its definition, types, sources (natural and anthropogenic), and various control techniques. It classifies air pollutants by origin, chemical composition, and state of matter, highlighting the impact of industrial processes and human activity on air quality. Additionally, it covers air quality indices, the dynamics of indoor air pollution, and methods for controlling sulfur oxides emissions.