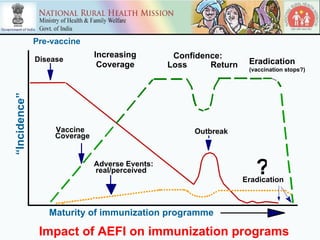
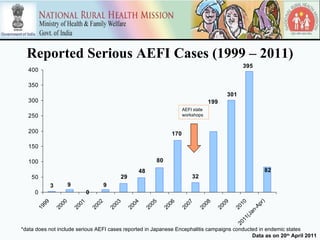
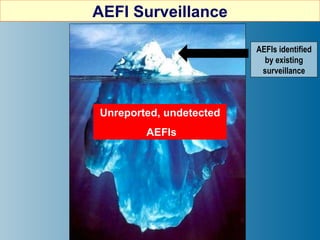
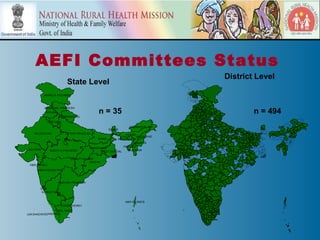
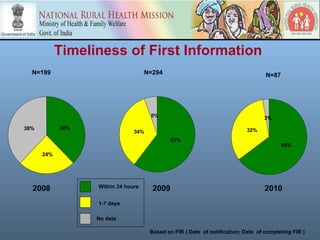
This document discusses adverse events following immunization (AEFI) in India. It provides background on AEFI surveillance in the country and outlines the national guidelines for AEFI case investigation, recording, and reporting. It also describes the composition and roles of the national, state, and district AEFI committees responsible for monitoring, investigating, and responding to AEFI cases in India. These committees are multidisciplinary and include representatives from health departments, medical colleges, and partner organizations. The document presents data on reported serious AEFI cases in India from 1999 to 2011 and discusses the impact of AEFI on immunization programs.