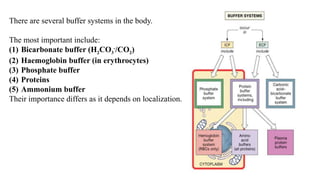
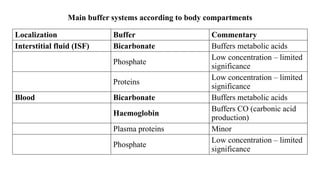
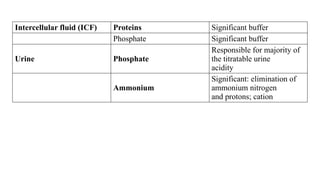
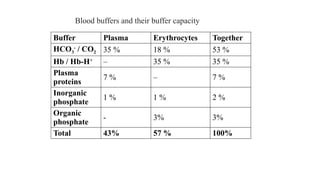
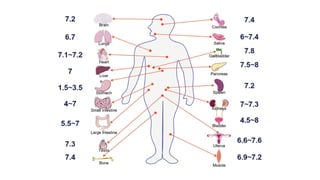
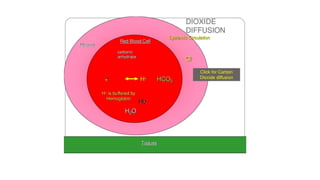
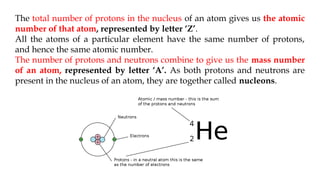
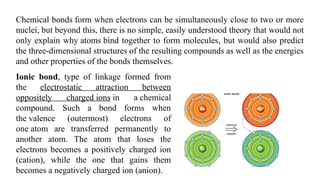
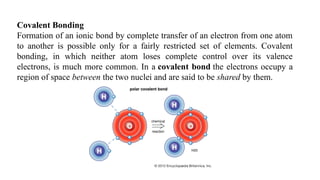
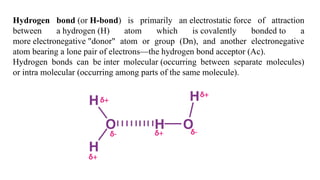
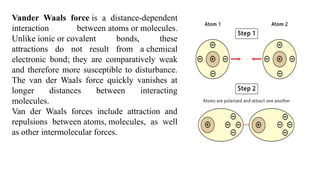
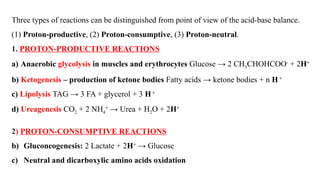
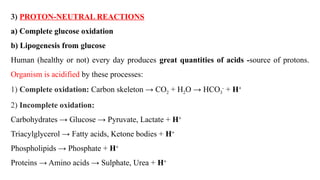
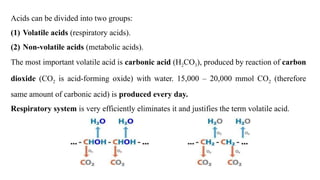
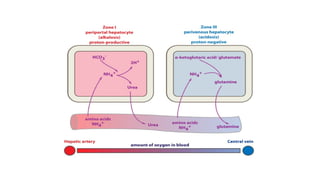
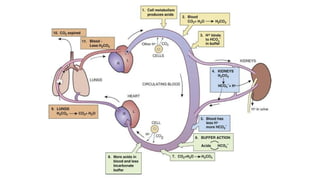
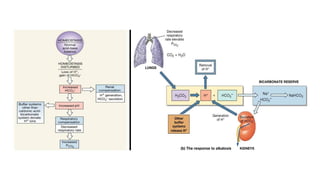
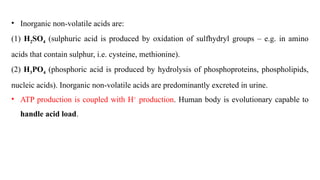
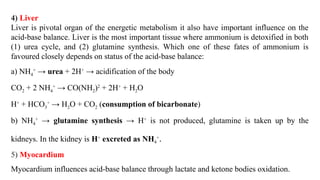
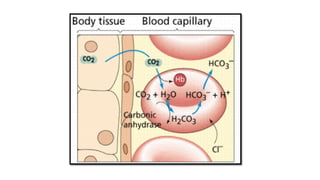
The document discusses the importance of acid-base balance in the human body, highlighting processes that regulate pH levels, including respiration and kidney function. It describes the production and elimination of acids and bases, differentiating between volatile and non-volatile acids and detailing the role of various buffer systems in maintaining homeostasis. The interactions between metabolic processes and acid-base equilibrium are outlined, emphasizing the significance of proper pH for physiological functions.




![Buffering systems
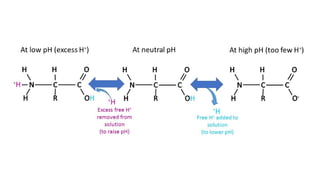
Buffers are substances capable of releasing and binding H+
. Short-term and acute changes
in acid-base balance can be balanced by buffers. Each buffer keeps its particular pH.
This pH could be calculated by means of the Henderson-Hasselbalch equation:
pH = pK + log [conjugated base]/[acid]
Henderson-Hasselbalch equation for bicarbonate buffer (HCO3
-
/CO2):
pH = pK H2CO3 + log ([HCO3
-
] / [H2CO3])
pH = pK H2CO3 + log ([HCO3
-
] / α x pCO2)
pH = pK ± 1 is range where buffers work optimally.
The ratio in bicarbonate buffer is 20:1 (HCO3
-
: CO2)](https://image.slidesharecdn.com/phinbodyandbuffersystem-241009072447-93ae78f1/85/Acid-base-balance-in-human-body-and-buffer-system-16-320.jpg)