Embed presentation

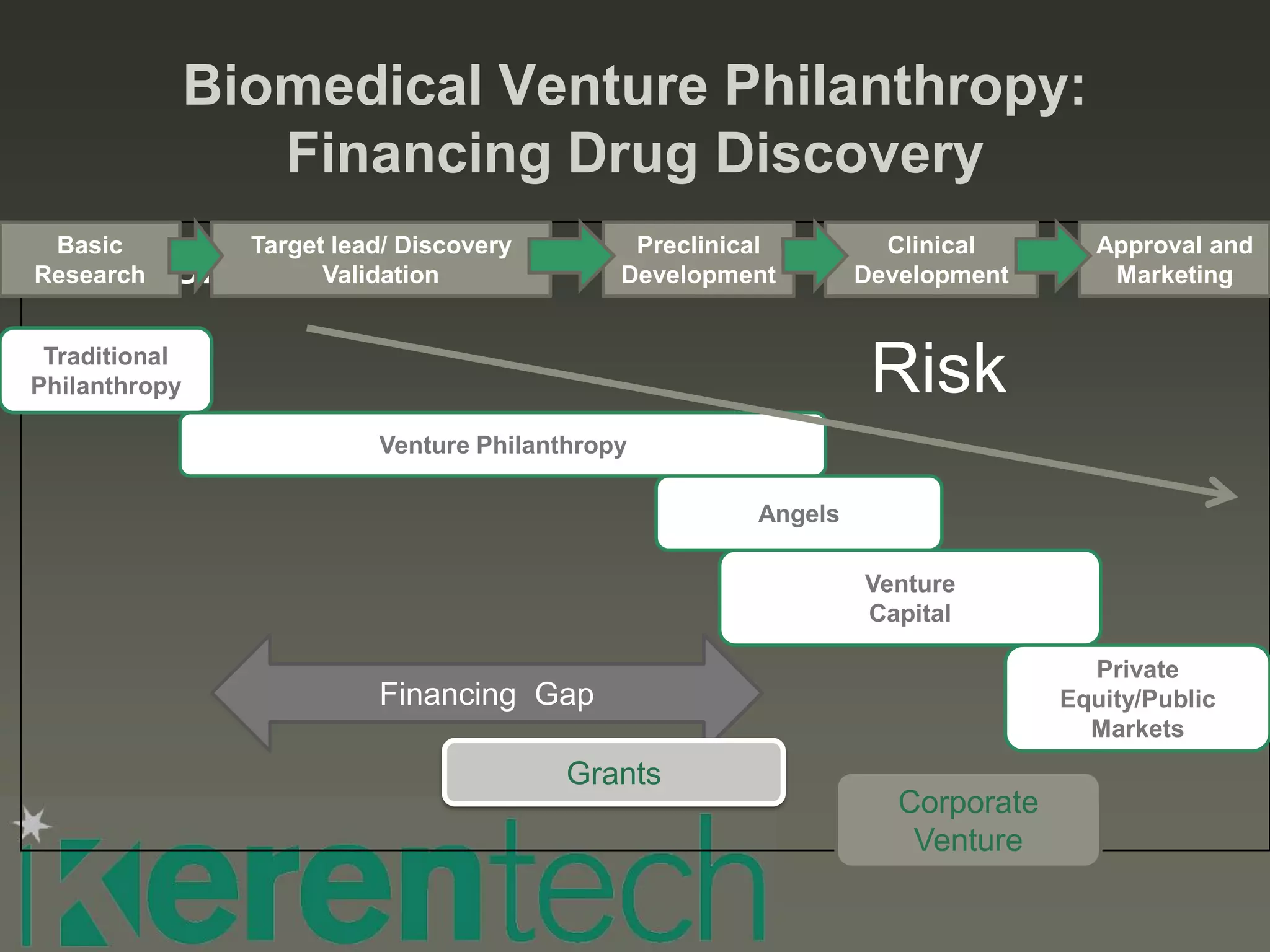














This document discusses considerations for biotech and medtech fundraising. It outlines the stages of risk in biomedical ventures and sources of financing at each stage, from traditional philanthropy to venture capital and public markets. It provides guidance on criteria that investors evaluate, such as technology/innovation, market opportunity, team experience, intellectual property, data, operational plans, regulatory pathways, valuation, and key success factors. The document emphasizes the importance of addressing real market needs with innovative solutions, having an experienced team and realistic operational plans, and securing funding well in advance of needs to support development programs.














