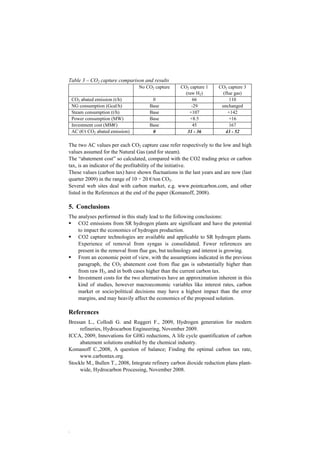
This document discusses hydrogen production via steam reforming with CO2 capture. It examines the possibilities of capturing CO2 from a steam reforming hydrogen plant. There are three main locations where CO2 can be captured: 1) from the raw hydrogen stream before purification, 2) from the purge gas stream after purification, and 3) from the steam reformer flue gas. Capturing from the raw hydrogen and flue gas streams can achieve overall CO2 removal rates of 60% and 90%, respectively. Amine-based capture is commonly used for the raw hydrogen and flue gas streams. A case study found the cost of capturing from the flue gas to be higher than from the raw hydrogen stream, and in both cases the