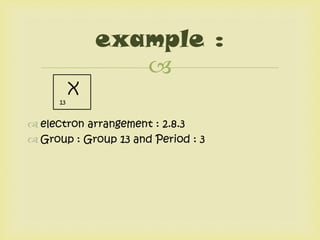
This document summarizes the history and development of the periodic table. It discusses early scientists like Lavoisier who first classified elements, Dobereiner who grouped elements into triads, and Newlands who saw a periodic pattern. Meyer and Mendeleev created early periodic tables that grouped elements by properties and left spaces for undiscovered elements. Moseley later determined that atomic number, not mass, was the basis for the recurring properties and rearranged the periodic table accordingly. The modern periodic table arranges elements in order of increasing proton number, with elements of similar properties in the same group and period based on electron configuration.